Superalloys 2000: Sample Abstract
9TH INTERNATIONAL SYMPOSIUM ON SUPERALLOYS
September 17-21, 2000 · Champion, Pennsylvania
 The 9th International Symposium on Superalloys, or Superalloys 2000, will be held from the evening of Sunday, September 17, until noon on Thursday, September 21, 2000, at the Seven Springs Mountain Resort in Champion, Pennsylvania. It is sponsored by the Seven Springs International Symposium Committee in cooperation with TMS, the TMS High Temperature Alloys Committee, and ASM International.
The 9th International Symposium on Superalloys, or Superalloys 2000, will be held from the evening of Sunday, September 17, until noon on Thursday, September 21, 2000, at the Seven Springs Mountain Resort in Champion, Pennsylvania. It is sponsored by the Seven Springs International Symposium Committee in cooperation with TMS, the TMS High Temperature Alloys Committee, and ASM International.
The basis of initial acceptance of a paper proposed for presentation at the meeting and publication in the proceedings volume is an extended 1,000-word abstract. The abstract must include a statement of purpose and describe the experimental procedure, results, and relevant conclusions. To aid in evaluation, potential authors are encouraged to include one or two figures in the abstract. Use of SI units is preferred. For reference, the following sample abstract is provided. For additional detail, see the call for papers.
In submitting the abstract, attach a cover page that contains the following information (in the order shown):
- Abstract Title
- Primary Author Name
- Primary Author Address
- Primary Author Phone
- Primary Author Fax
- Primary Author E-mail
- Co-Author Names
Mail the cover page and abstract to
The abstract submission deadline is September 23, 1999; authors will
be notified of acceptance by November 19, 1999; camera-ready manuscripts
must be delivered to the Program Committee by February 25, 2000.
A New Type of Microstructural Instability in Superalloys--SRZ
W.S. Walston, J.C. Schaeffer and W.H. Murphy
GE Aircraft Engines, Cincinnati, OH
A new type of microstructural instability has been found in superalloys containing a high rhenium content. This instability has been termed secondary reaction zone (SRZ) because it typically occurs beneath the diffusion zone of coatings that are typically applied to superalloys. This same type of instability also can occur in the microstructure of the alloy and is called cellular colonies in order to differentiate it from the SRZ beneath coatings. The SRZ and cellular colonies form a three-phase constituent with a g' matrix containing g and P phase (TCP) needles. The interface between these constituents and the normal g/g' microstructure is very weak, and cracking along this interface can severely reduce properties.
SRZ is similar to previously observed constituents in other alloy systems.1,2 These constituents form by cellular or discontinuous precipitation. Cellular precipitation results from a supersaturated parent phase (a') decomposing into a new phase, b, and a structurally identical phase, a. The classic cellular precipitation has been studied extensively in simple alloy systems with nucleation occurring at grain boundaries. SRZ has a lamellar structure, which grows in colonies that fan out radially from the initiation site. SRZ resembles cellular recrystallization commonly observed in superalloys,3 however the mechanisms of formation and morphology of SRZ are different.
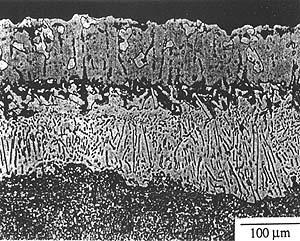
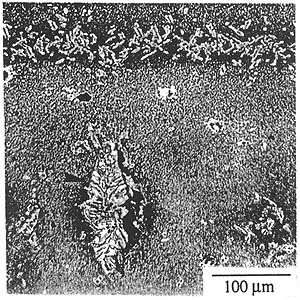
The SRZ discovered in Re-containing superalloys involves the conversion of a g matrix with g' precipitates to a g' matrix containing g and P phases. SRZ differs from classical cellular precipitation in that nucleation occurs at sites other than grain boundaries, such as coating/substrate interfaces, dendrite cores and internal precipitates. SRZ refers to the instability reaction under coatings, while cellular colonies refer to the instability reaction at grain boundaries or in dendrite cores. Figure 1 shows an example of the instability phenomena at each of these nucleation sites.
SRZ was first observed in experimental, third generation single crystal superalloys under aluminide and platinum aluminide coatings. One of these alloys, Alloy 5A,4 contains 6.25 wt.% Re and precipitates a small amount of TCP at temperatures of about 2000oF, however this did not affect properties. The occurrence of SRZ under coatings after high temperature exposure was unexpected and never before observed in superalloys. The SRZ occurs beneath the normal diffusion zone of the coating, hence the name secondary reaction zone (SRZ). Due to the propensity of the SRZ interface to crack, along with the reduced specimen cross-sectional area, SRZ beneath coatings can reduce properties.
This same type of instability has also been observed in dendrite cores of single crystal superalloys in the absence of coating diffusion gradients or grain boundaries. It is believed that the segregation of Re to the dendrite cores during solidification leads to an extremely unstable g phase resulting in cellular colony nucleation from a heterogeneous site in the dendrite core. A large number of rupture tests were performed on Alloy 5A at temperatures up to 2000oF without any unusual data. However, in many of the subsequent longer time tests, results were obtained that were as low as 30% of the expected rupture life, as shown in Figure 2. Detailed metallographic examination of these specimens showed that cellular colonies in the dendrites were present in large amounts on and adjacent to the fracture surface. The unexpectedly low results only occurred in a small percentage of the rupture tests performed, however the effect of the cellular colonies was very dramatic when it did occur. These observations led to composition changes resulting in a new alloy, Rene N6.5
Several studies have been conducted to understand, predict and eliminate the presence of SRZ and cellular colonies in Re-containing superalloys. SRZ lends itself to interpretation with classical nucleation and growth theory. The basic equation describing homogenous nucleation is:
DG = n(DGa/a' + DGe) + hgn2/3
where DG is the free energy for an SRZ nuclei, DGa/a' is the free energy difference between parent and product phases per unit volume (supersaturation), DGe is a strain energy term, h is a shape factor, g is the surface free energy between the phases and n is a volume term. Nucleation is controlled by a number of factors, including supersaturation, surface energy, strain energy and the number of heterogeneous sites. Supersaturation can occur either by external (coating) or internal (segregation) chemistry imbalances.
The effect of surface and strain energy was studied by modifying the surface condition of specimens prior to coating. It was found that processes that imparted residual stress to the surface promoted the formation of SRZ. The effect of stress was also demonstrated by observation that higher angle grain boundaries promoted the formation of cellular colonies.6 Growth of SRZ is a diffusion controlled process. Measurements of SRZ growth at 2000oF show the interdiffusion coefficient for SRZ to be very close to that of Ni in g and g'.
Two tests have primarily been used to determine an alloy's propensity to form SRZ and cellular colonies. The first test evaluated the cellular colonies that are formed away from the coating. As-cast, single crystal specimens were used to accentuate the amount of segregation. After exposure at 2000oF, the specimen was examined for the presence of cellular colonies in the dendrites. If no cellular colonies were observed, then it was believed that the alloy would not form cellular colonies in service, especially after a proper solution heat treatment. The second test evaluated the alloy's propensity to form SRZ beneath a coating. Specimens were given different surface preparations as a means to also study the effect of surface stress. Following the coating application and exposure at 2000oF, the amount of SRZ beneath the coatings was measured.
Based on these tests, a database was created to develop predictive models for SRZ and cellular colony formation as a function of alloy chemistry. From these models, it has been learned that Re is the largest factor in the propensity to form these instabilities. W also contributes to their formation, but interestingly the other refractory elements, Cr and Mo, are actually beneficial in preventing SRZ beneath coatings. This may be due to the interaction between TCP and SRZ formation. The g' hardeners, Al, Ta and Ti, were not significant. Simple regression analysis for SRZ beneath the coating yielded the following relationship (at.%):
[SRZ]1/2 = 13.88 (Re) + 4.10 (W) - 7.07 (Cr) - 2.94 (Mo) - 0.33 (Co) + 12.13
The prevention of SRZ and cellular colonies in Re-containing superalloys involves the use of several different techniques. The easiest method is to simply lower the Re content below a threshold value for that alloy system, however this often results in unsatisfactory creep rupture strength. A proper balance of alloying elements using the predictive models can be achieved, as demonstrated by the development of Rene N6. Other methods include heat treatments to reduce segregation, stress relief techniques and coating parameter adjustments.
Perhaps the most effective technique to eliminate SRZ under coatings is to tie up refractory elements through local carburizing.7 Extremely fine, stable carbides can be introduced to the surface layer by a means such as chemical vapor deposition followed by a diffusion cycle. This ties up many of the refractory elements in the surface layer, effectively removing the chemical driving force to form SRZ. It has been shown that this carburization technique completely eliminated the SRZ layer underneath an aluminide coating on Rene 162 following a 2000oF exposure. These methods to prevent SRZ and cellular colonies are key to developing high Re-containing superalloys that are needed in today's advanced aircraft engines.
1. D. Turnbull, Acta Met., Vol. 3, (1955), pp. 55-62.
2. B.E. Sundquist, Metall. Trans., Vol. 4, (1973), pp. 1919-1934.
3. J.M. Oblak and W.A. Owczarski, Trans. TMS-AIME, Vol. 242, (1968), pp.
1563-1568.
4. C.M. Austin, R. Darolia, K.S. O'Hara and E.W. Ross, U.S. Patent
5,151,249, (1992).
5. W.S. Walston, E.W. Ross, K.S. O'Hara and T.M. Pollock, U.S. Patent
5,270,123,(1993).
6. T.M. Pollock, Mat. Sci and Eng. B, Vol. B32, (1995), pp. 255-266.
7. J.C. Schaeffer, U.S. Patent 5,334,263, (1994).
Figure 1. Instability in high Re-containing single crystal superalloy. (a) SRZ under aluminide coating, (b) cellular colonies along low angle boundary, (c) cellular colony in dendrite.
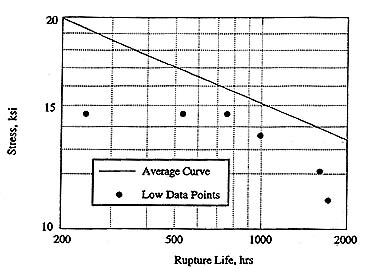
Figure 2. 2000oF average rupture strength curve of alloy 5A. Low points occurred due to the presence of cellular colonies in the microstructure.
The content of this site was developed by Randy Bowman (randy.bowman@lerc.nasa.gov); your feedback is welcome.
 The 9th International Symposium on Superalloys, or Superalloys 2000, will be held from the evening of Sunday, September 17, until noon on Thursday, September 21, 2000, at the Seven Springs Mountain Resort in Champion, Pennsylvania. It is sponsored by the Seven Springs International Symposium Committee in cooperation with TMS, the TMS High Temperature Alloys Committee, and ASM International.
The 9th International Symposium on Superalloys, or Superalloys 2000, will be held from the evening of Sunday, September 17, until noon on Thursday, September 21, 2000, at the Seven Springs Mountain Resort in Champion, Pennsylvania. It is sponsored by the Seven Springs International Symposium Committee in cooperation with TMS, the TMS High Temperature Alloys Committee, and ASM International.