 |
53 (1) (2001), pp. 28-33 |
|---|
Lead-Acid Batteries: Overview
 |
53 (1) (2001), pp. 28-33 |
|---|
|
TABLE OF CONTENTS |
|---|
|
|
Oxygen-recombination chemistry has been wedded to traditional lead-acid battery technology to produce so-called sealed, or valve-regulated, lead-acid products. Early attempts to incorporate recombination into lead-acid batteries were unsuccessful because of excessive cost, size, and/or complexity, and none were effectively commercialized. Over the past 20 years, recombination systems have been developed and are undergoing an extensive program of definition and refinement at many battery companies. This paper presents the basic chemistry of oxygen recombination in lead-acid cells and briefly compares it with the more highly developed nickel-cadmium system, which also operates on the oxygen cycle. Aspects of gas and thermal management relevant to valve-regulated lead-acid batteries are discussed in some detail.
The first fully functional, commercially viable recombinant
lead-acid products came on the market in the early 1970s. However, some of the
principles necessary for such a technology were known long before this. For
example, the gelling of sulfuric acid with silica was proposed in the late 1800s,1
and eventually led to the development of gelled-electrolyte lead-acid batteries.2
Gelled sealed cells were reportedly manufactured as early as 1934 by Elektrotechnische
Fabrik Sonneberg in Germany,3
but apparently on a very limited basis.
Thomas Edison first proposed the principle of gas recombination within a battery
in 1912;4 and over the
next 60 years various attempts were made to commercialize this concept for the
lead-acid couple.5 Most
approaches were not successful because of excessive cost, bulk, and/or complexity—or
they just did not work.
In the late 1960s, a number of prominent lead-acid battery companies had development
programs directed toward producing a viable sealed battery, spurred by the successful
commercialization of nickel-cadmium technology during the previous two decades.
It was clear that the chemistries were very similar, but the key stumbling block
was the amount of electrolyte necessary in the lead-acid system to realize acceptable
discharge capacities and still have sufficient void volume within the cell to
facilitate oxygen recombination. This dilemma was solved by the development
of a glass microfiber separator, which has the ability to hold large quantities
of electrolyte and, at the same time, has a porosity in excess of 90%. About
27 years ago, Gates came out with the first fully commercialized product line.
Since then, dozens of other companies have followed suit, and, today, valve-regulated
lead-acid (VRLA) batteries are recognized as a new, significant technology.
This paper outlines some of the more obvious chemical differences between flooded
and recombinant lead-acid systems and poses several speculative mechanisms that
may be operative in VRLA batteries but are far from proven.
|
|
||||||||||||||
|
BASIC CHEMISTRY
The chemistry occurring
at the positive plate on charge and overcharge is identical to what
would take place in a flooded system. The primary overcharge reaction,
electrolysis of water, takes place with the evolution of oxygen gas
and an increase in the acidity of the electrolyte within the pores if
diffusion is restricted:
As the overcharge process continues, a greatly sim-plified view of what is taking place would involve hydro-nium- ion diffusion away from the plate to minimize the concentration gradient and oxygen diffusion against virtually an infinite gradient. In a properly engineered recombinant cell, the positive plate contains pores with only a thin film of electrolyte in them, estimated to be 0.01 mm thick. This clearly limits three-dimensional diffusion paths for hydronium ions and somewhat re-stricts the liquid transport. Oxygen transport, on the other hand, is facilitated by this thin-film condition, as the diffusion coefficient in the gas phase (~0.2 cm2 /s)6 is considerably greater than that in typical sulfuric-acid electrolyte (9 ´ 10-6 cm2 /s),7 resulting in a mass-transport rate difference of about ten when oxygen solubilities are factored. The oxygen generated at the positive diffuses principally through the void spaces in the separator toward the negative plate, which will typically be only about 1–2 mm away. The apparent diffusion coefficient will vary with factors such as the separator saturation level, and tortuosity,8 showing optimal oxygen transport below about 80% saturation level; above 90% saturation, it has been reported that the glass microfiber separator behaves as if it were flooded.8 Because the fibers are randomly oriented and thickness/grammage relationships vary from one pa-per- making process to another, even at a fixed satura-tion level, oxygen transport may vary considerably among separator samples. Still, unless the separator is saturated, oxygen transport to the negative electrode is relatively rapid and is not seen as the rate-limiting step in the overall oxygen-transport process. The rate-limit-ing step appears to be diffusion through the electrolyte film in the negative plate pores so that the oxygen can react with the sponge lead of the negative plate, as shown conceptually in Figure A. This film thickness, estimated to be about 0.1 mm in a typical VRLA cell,9 can and will vary substantially with changes in cell materials and construction, manufacturing tolerances, and any other factors affecting electrolyte distribution. With fixed parameters, film thickness may even vary from one area of the negative plate to another. To ensure that gassing is minimal in these cells, most VRLA products have material balances such that the negative electrode is overbuilt relative to the positive; thus, there will always be an excess of lead sulfate along with the sponge lead, which reacts with electrogenerated oxygen. Between these two conditions, the negative should not go into hydrogen evolution except under conditions of overcharge where the ability of the cell to recombine all the O2 generated is exceeded. The oxygen-recombination process is written in the following way, but there is considerable disagreement over whether it is largely chemical or electrochemical in nature:
Reaction B is a gas/solid reaction and should be kineti-cally hindered, but it is occurring in a liquid phase, so the energetics are uncertain. Summing Reactions B–D gives the overall recombination reaction, which should also occur directly as a purely electrochemical process:
This has recently been postulated as the actual mechanism,10 but the net result either way is the same. Hydronium ion is consumed and water is generated in the pores of the negative plate. Note that Reaction E is the opposite of the positive-plate overcharge (Reaction A) and, thus, there appears to be no net change in the chemistry of the cell. How-ever, quite a bit has, in fact, taken place. Acid has been generated in the pores of the positive plate and electrogenerated oxygen has diffused to the negative plate through a partially saturated separator and thin electrolyte films on both plates. The oxygen has reacted with the acidic electrolyte to reform the water electrolyti-cally, generating water in the pores of the negative plate. Although no net chemical change has taken place in the cell, electrical energy will have been converted to heat. Additionally, if some portion of the negative elec-trode goes into overcharge, hydrogen gas will be gen-erated via the following simplified reaction:
This will further diminish the acidity in the negative plate and, again, free diffusion conditions are necessary to maintain the chemical environment in a balance state. Changes in the acidity in both plates at the interface area with the electrolyte can have a profound impact upon the precipitation/dissolution equilibria of lead-sulfate species and, thus, may directly affect the plate morphologies. The chemistry involved in the overcharge processes is considerably more complex than this, with many minor secondary reactions which are not directly related to oxygen recombination taking place.11 In addition, on overcharge and discharge, extremely complex chemis-try apparently takes place at the grid/active-material interface of the positive plate12-15 . That chemistry is not discussed in this paper, nor is any attempt made to thoroughly describe the various processes taking place that may affect the overall cell oxygen, hydrogen, and charge balances. Instead, the focus is on the gas recombination chemistry and some of the ways battery technologists must deal with it in developing functional VRLA products. |
Sealed nickel-cadmium cell technology has been developed to optimize the efficiency of the oxygen-recombination process. The chemistry is such that the cells can be operated in a starved condition (relative to VRLA systems) and under normal operating conditions, there is no venting of gases because the cells have a thin, oxygen-permeable separator with a high void volume and an overbuilt active cadmium-negative electrode with a thin electrolyte film. Unlike the lead-acid system, the primary function of the electrolyte is to provide good conductivity within the cell and only water is involved in the overall cell reaction, leaving the KOH electrolyte relatively unchanged during charge/discharge cycling. Table I shows the chemistries side by side, and Table II compares some of the critical cell design characteristics. (Most of the numbers in Table II are estimated and are only intended to give an overall picture of how the two technologies compare.) Sealed nickel-cadmium cells do have safety vents which will release gas in the event of a pressure buildup, but they are normally intended to operate at very high internal pressures with minimal gassing. The positive plate is designed to go into overcharge first, thus generating oxygen, and transport to and recombination at the negative is promoted. Because it is overbuilt relative to the positive and constantly being oxidized by oxygen, the cadmium electrode does not normally reach a potential where hydrogen is generated. This is also facilitated by a carefully controlled, narrow fill-weight range that is great enough to provide good conductivity and small enough so the separator and plate pores are not flooded, which would lead to a pressure buildup. Because no gases are usually given off, all of the overcharge current goes into heat generation. Therefore, charging and thermal management are critical issues; only constant-current charging is recommended for nickel-cadmium cells and only at moderate and low continuous levels, about C/3 at most.
|
Table I. Comparison of Nickel-Cadmium
and Lead-Acid Chemistries
|
||
|
Nickel-Cadmium Chistry
|
Lead-Acid Chemistry
|
|
| Negative |
Cd(OH)2(s) + 2e-
|
PbSO4(s) + 2e- + H+
|
| Overcharge |
2H2O
+ 2e-
|
2H+ + 2e-
|
| Positive |
Ni(OH)2(s) + OH-
|
PbSO4(s) + 2H2O
|
| Overcharge |
4OH-
|
2H2O
|
| Overall Cell Process |
Cd(s)
+ 2NiOOH(s) +2H2O
|
Pb(s)
+ PbO2(s) + 2H2SO4
|
| Recombination Reaction |
2Cd(s)
+ O2 + 2H2O
|
2Pb(s)
+ O2 + 2H2SO4
|
|
|
||
Cell-to-cell balance in batteries is also a major concern,
since imbalances could drive one or more cells in a battery into reversal, thus
causing damage and possibly resulting in hydrogen generation at the positive
nickel electrode and oxygen at the negative. The oxygen will eventually recombine
but the hydrogen will lead to pressure buildup. The consequences of this are
obvious and can be minimized somewhat through modification of the cell chemistry,
but the predominance of single-cell manufacture in sealed nickel-cadmium (with
attendant sorting by discharge capacities and other performance attributes)
attests to the seriousness of this limitation. Sealed nickel-cadmium applications
manuals are also dominated by charging systems, temperature sensing, pressure
and thermal management considerations, and other factors related directly to
the oxygen-recombination process. It is addressed at length because it is a
two-edged sword, giving the technologist a tool to allow for the construction
of sealed power systems but also wreaking extreme havoc if this tool is not
controlled and applied properly. It should be pointed out that nickel-cadmium
cells do generate hydrogen on normal over-charge and do gas, but these occurrences
are minor compared to VRLA systems.
|
Table II. Comparison of Nickel-Cadmium
(Ni-Cd) and Lead-Acid Construction Attributes, Electrolyte Distribution
|
||
|
Parameter/Cell Dimension
|
Sealed Ni-Cd
|
Sealed Lead-Acid
|
| Separator Thickness |
<1 mm
|
1-2 mm
|
| Separator Material |
Nylon or polyprolylene
|
Glass microfiber
|
| Separator Porosity (%) |
85-95
|
85-95
|
| Electrolyte Volume (cm3/Ah) |
~4
|
~8-10
|
| Electrolyte in Separator (%) |
~10
|
~75
|
| Electrolyte in Plates (%) |
~90
|
~25
|
| Saturation Level of Separator (%) |
20-30
|
80-90
|
| Saturation Level of Negative Plate (%) |
70-80
|
50-60
|
| Total Cell Pore Filling (%) |
50-60
|
70-90
|
| Negative Plate Film Thickness (mm) |
~0.003
|
~0.1
|
| Positive Plate Film Thickness (mm) |
~0.01
|
~0.01
|
| Electrolyte Composition |
~7 M KOH
|
~5 M H2SO4
|
| O2 Diffusion Coefficient in Electrolyte (cm2/s) |
6 ´ 10-6
|
9 ´ 10-6
|
|
|
||
Why dwell on nickel-cadmium technology? Because much of what
has had to be done for nickel-cadmium in the past is the future of VRLA batteries
and cells. The latter are just starting up the learning curve, and, because
of the similarities in the chemistries, a great deal can be learned from the
problems that have befallen nickel-cadmium cell developments and the solutions
that have arisen. Fortunately, at this point it appears that the sealed lead-acid
system is more forgiving, primarily because it does experience moderate gassing.
As applications demands push the industry to achieve greater performance levels
in more varied and hostile environments, however, VRLA technology may experience
problems similar to those of nickel-cadmium batteries.
Descriptions of the oxygen cycle functioning in sealed lead-acid systems sounds
like descriptions of a nickel-cadmium cell: the positive goes into over-charge,
liberating oxygen, which readily diffuses to the surface of the negative, where
it is recombined. Between this and the presence of excess negative active material,
the potential at this plate never rises to a level where hydrogen is generated.
The overall chemistry shows no net change, there are no gases given off, and
all the electrical energy is converted to heat, which presumably is readily
dissipated.
However, it does not entirely work this way very often, if ever. Sealed-lead
technology is in its infancy, and it does not have an aerospace connection or
DOE funding to promote detailed scientific studies as was the case with other
chemistries. We do not know as much as we should about plate morphologies, film
thicknesses, separator structures, and critical process parameters such as fill
weights and electrolyte distribution, just to name a few.
All VRLA cells and batteries currently being manufactured give off relatively
small quantities of gases under some conditions, and not just abusive situations.
Carbon dioxide is liberated from slow neutralization of lead carbonates and
from chemical or electrochemical oxidation of organics present in expanders,
other paste additives and/or separators. CO2
is usually generated slowly and steadily early in the life of a cell; other
gaseous breakdown products such as methane may also be generated and vented.
The primary gases of note are hydrogen and/or oxygen because of obvious concerns
if they are vented in certain proportions and a spark source is present. Oxygen
will recombine at the negative up to a current density reflective of the ability
of the cell design to accomplish this; if the internal cell pressure then exceeds
the valve-release level, some oxygen will be vented from the cell. Hydrogen
is more commonly given off, even at very low overcharge levels characteristic
of float applications; although the amounts are detectable they are insignificant
relative to flooded batteries due to the extremely low Coulombic efficiencies
involved. Theoretically, hydrogen can also recombine within the cell, being
either consumed at the positive, much as oxygen is at the negative or catalytically
reacting with oxygen directly. This does not appear to take place under normal
operating conditions. Figure 1 depicts
the variation in gas composition and total gas vented in an over-charge process.
Figure 2 shows internal gas composition
variations during a 24-hour taper-current formation of a spiral-wound 2.5 ampere
hour (Ah) cell.
These data raise many questions, but the one most pertinent to this discussion
is why H2 is seen under all conditions, both
within the cells and vented. It appears that some areas of the negative plate
are in overcharge and generating hydrogen while others are efficiently recombining
oxygen. This follows from a conceptual model of the glass separator which has
oxygen transport taking place through relatively large gas channels, or pores,
and the other areas of the glass mat being saturated with electrolyte. Areas
of the negative plate that these channels have access to will be the recombination
sites and those plate surfaces that face flooded separator pores, especially
those with fine pore structures themselves, will go into overcharge and generate
hydrogen. In order for this to be true, the negative-plate potential will be
dominated by one or the other of these processes, or be a sum of their contributions.
This has been observed for other types of porous electrodes6
and may readily explain this phenomenon.
Figure 3 shows three idealized cases
for cell-potential excursions during constant- current charge and overcharge;
negative-plate values will track these trends at different voltages. Curve A
is for a cell that has its separator saturated with electrolyte; upon reaching
a full state of charge it goes into hydrogen gassing and stays there because
recombination is inefficient. Curve C depicts the overcharge behavior of a cell
with extremely good oxygen recombination; the negative plate is almost completely
depolarized and the cell cannot achieve a potential where hydrogen gassing will
occur. All of the overcharge current is being converted to heat. Not incidentally,
the negative plate cannot be fully recharged in this case.
Curve B shows a cell that initially goes to hydrogen gassing, but, as the oxygen-recombination
process begins to dominate, the negative-plate voltage is dragged down and the
gassing rate diminishes. However, even when the cell is recombining at a cell
voltage of about 2.4 V–2.5 V, most of the gas given off is hydrogen. A mixed
potential situation exists that is balanced between H2
evolution and O2 recombination, depending
upon the relative contributions of the Pb/PbSO4
and H2/H+
couples.
The parameters affecting this mixed-potential condition are unclear at present.
Factors such as the charge current level and basic cell design are obvious,
but it is possible to see the same cell just off formation go from Curve C behavior
to Curve B within two or three C/5 discharges and, in some cases, approach Curve
A; similar dramatic changes in recombination efficiency have been seen for the
cell at the C/10 charge rate, although it may be a case of incomplete formation
or some other anomaly. What can change so much within a few cycles? Very small
weight losses are involved, so that will not induce a significant change in
total void volume. If the negative is not formed, there will be some fluid volume
change associated with sulfate conversion from PbsO4
to H2SO4
, but it seems unlikely that this would create the changes seen. Within the
first few charge/discharge cycles, the surface area and/or plate morphology
of thenegativecould changeandthiswould have a direct impact on film thickness.
What seems more likely is that the electrolytemoves betweentheseparatorand the
plates and/or within these materials. For a given surface area, moving electrolyte
from the negative plate into theseparator, possiblyduetohydrogen- gas generation,
will decrease the film thickness in the plate pores, but it will also decrease
the void volume in the separator. If O2 cannot
get to the negative plate, the film-thickness effect upon recombination efficiency
is academic.
In other cases, as pressure builds in the cell, electrolyte may be physically
moved out of the separator in some areas with the largest pores and be pumped
into the headspace or other separator areas, creating more or selective void
volume for enhanced oxygen transport. Although the separator model (depicted
in the sidebar and elsewhere8)
shows discrete gas paths directly connecting the plates, it may be that the
actual separator/electrolyte structure is fairly random, with oxygen molecules
diffusing throughvarious combinationsofgasand liquid phases. The distinction
between this and highly tortuous, continuous gas paths would be slight; both
may exist.
It should be pointed out that this mi croscopic view of the plate/separator
structure is not inconsistent with the original concept of direct plate-to-plate
recombination. Sufficient void volume must exist in the separator to facilitate
oxygen transport to the negative and, in a macroscopic sense, the electrolyte
is uniformly distributed throughout the negative plate surface with a thin-film
condition necessarily existing, again to support the oxygen cycle. The existence
of some microscopic areas of the negative plate in a flooded condition, and
thus generating hydrogen on overcharge, will normally not disrupt the oxygen
cycle, but appears to coexist.
|
Table III. Float Voltage and Gassing*
Characteristics in a 24 V/5.0 Ah Cell String Floated at 2.35 V/Cell
|
|||||
|
Float Voltages (mL Gas), Time on Float
|
|||||
|
Cell Number
|
Pre-Float OCV, V
|
0 h
|
16 h
|
24 h
|
42 h
|
| 1 |
2.126
|
2.30
|
2.37
|
2.42
|
2.41
|
| 2 |
2.126
|
2.22
|
2.25
|
2.27
|
2.28
|
| 3 |
2.129
|
2.22
|
2.22
|
2.22
|
2.22
|
| 4 |
2.132
|
2.24
|
2.24
|
2.29
|
2.31
|
| 5 |
2.132
|
2.57 (0.0)
|
2.48 (12)
|
2.45 (21)
|
2.43 (35)
|
| 6 |
2.135
|
2.21
|
2.23
|
2.22
|
2.23
|
| 7 |
2.135
|
2.24
|
2.26
|
2.37
|
2.39
|
| 8 |
2.139
|
2.38
|
2.46
|
2.35
|
2.38
|
| 9 |
2.140
|
2.55 (0.0)
|
2.24 (50)
|
2.26 (55)
|
2.26 (57)
|
| 10 |
2.140
|
2.38
|
2.51 (8)
|
2.48 (26)
|
2.45 (46)
|
| 11 |
2.140
|
2.36
|
2.48 (10)
|
2.45 (24)
|
2.24 (36)
|
| 12 |
2.142
|
2.38
|
2.48
|
2.43
|
2.41
|
| Float Voltage Variation (mV) |
36
|
29
|
26
|
23
|
|
|
* Gas composition is exclusively H2 and CO2. |
|||||
Electrolyte-fill volume is critical with VRLA products, requiring an amount great enough to provide the desired dis charge capacity and saturate the separator at an 80–95% level, yet small enough so that the separator is not fully saturated and free electrolyte (in capillary contact with the separator) does not exist to any significant extent within the cell. Small differences in fill weights cell to-cell could cause imbalances in top-of-charge voltages, which is a shortcoming of recombinant systems. It seems that the desired operating range for recombinant cells is somewhere in between flooded and starved, yet this area is the one where apparently insignificant changes in cell materials and amounts can be translated into widely different recombination behaviors. It appears that in this region, the cells are in complex, dynamic situations where hydrogen generation and oxygen recombination are taking place simultaneously on different portions of the negative plate and very subtle changes in the cell environment can swing control of the plate (and thus the cell) potential from one process to another. In a float or cycling application with many cells in a series string or series/parallel array, it is fatuous to be lieve that all the cells will be at, or even near, the nominal volts-per-cell value. Table III shows data for a 24 V series string of cells floated at 2.35 V/cell, a voltage where minimal gassing would normally occur. In fact, several of the cells did gas and float voltages were widely variant, though they were con verging with time and this is a very short experiment relative to batteries in float service. TableIV shows longer-term data for a 300 Ah cell battery in actual float service over an extended period of time; in fact, the variation is even more sub stantial and individual cell voltages vary considerably.
|
Table IV. Individual Cell Voltage
Data for 300 Ah Prismatic Cells in a 48 V/600 Ah Array Floated at 2.28
V/Cell
|
||||
|
Cell
Number |
Original
Voltage |
Voltage at
30 Days |
Voltage at
78 Days |
Voltage at
106 Days |
| 2 |
2.25
|
2.25
|
2.22
|
2.24
|
| 4 |
2.25
|
2.31
|
2.42
|
2.37
|
| 6 |
2.27
|
2.25
|
2.24
|
2.24
|
| 8 |
2.26
|
2.25
|
2.24
|
2.24
|
| 10 |
2.31
|
2.27
|
2.27
|
2.26
|
| 12 |
2.26
|
2.29
|
2.38
|
2.31
|
| 14 |
2.26
|
2.24
|
2.23
|
2.23
|
| 16 |
2.27
|
2.21
|
2.18
|
2.20
|
| 18 |
2.26
|
2.24
|
2.22
|
2.22
|
| 20 |
2.32
|
2.29
|
2.30
|
2.31
|
| 22 |
2.26
|
2.32
|
2.18
|
2.20
|
| 24 |
2.29
|
2.24
|
2.23
|
2.23
|
| 26 |
2.25
|
2.31
|
2.32
|
2.25
|
| 28 |
2.25
|
2.37
|
2.39
|
2.34
|
| 30 |
2.27
|
2.28
|
2.32
|
2.40
|
| 32 |
2.32
|
2.22
|
2.14
|
2.15
|
| 34 |
2.27
|
2.25
|
2.15
|
2.22
|
| 36 |
2.27
|
2.22
|
2.28
|
2.24
|
| 38 |
2.29
|
2.28
|
2.28
|
2.28
|
| 40 |
2.26
|
2.26
|
2.22
|
2.24
|
| 42 |
2.27
|
2.24
|
2.22
|
2.23
|
| 44 |
2.27
|
2.22
|
2.17
|
2.22
|
| 46 |
2.30
|
2.27
|
2.30
|
2.34
|
| 48 |
2.23
|
2.22
|
2.20
|
2.22
|
| Range (mV) |
80
|
160
|
280
|
250
|
|
|
||||
It has been pointed out that float currents for VRLA cells are several times
greater than those for flooded vented analogs due to the depolarizing effect
of the oxygen-recombination process on the negative electrode,16
and the more efficient the latter the greater the disparity will be. If a cell
that is intended to be a recombinant product is overfilled, thus flooding the
separator, it will initially behave like a vented cell and will gas almost stoichiometric
volumes of hydrogen and oxygen. Eventually, it will achieve a starved configuration
and the gassingrate will sharply diminish.When this is achieved it will function
as a re combinant cell would, but at the price of having released relatively
large quanti ties of gases and, possibly, acid spray. Since many of these types
of products are put through a jar formation, overfill ingwillalso have obvious
process draw backs.
Underfilling will allow for very efficient recombination performance, but it
is not feasible for at least two reasons. Because the glass separator has such
a high affinity for electrolyte, achieving uniformacid distribution is difficult
even with normal fill weights; underfilling in the extreme will lead to dendrite
formation because of acid depletion at the fill-liquid front and subsequent
dissolution of PbSO4 and/or PbO in the alkaline-
fluid medium. This latter factor can be largely overcome with electrolyte additives,
but the effect of uneven electrolyte distribution is an open question. Discharge
capacity will also be curtailed at low fill weights, as most recombinant systems
aredesigned for 70–80% utilization levels of electrolyte. If the electrolyte
volume is reduced without increas ing the specific gravity, the utilization
levels may be pushed up to unaccept able values or discharge capacities may
diminish.
An underfilled condition may also be deleterious by being too much of a good
thing. When recombination is very ef fective, it will hold the negative plate
near the open-circuit value. If a cell is on a float voltage of, for example,
2.35 V, but its voltage is held down to 2.25 V by oxygen recombination, the
cell will draw high currents to try to get to 2.35V. All of this current is
being converted to heat, which will also promote a higher current draw; in the
extreme this condition can lead to thermal runaway if sufficient currents are
available and the cell cannot dissipate the heat being generated.
When VRLA cells or batteries are designed, the tendency is to try to build in
the most efficient level of recombination possible. Because of some of the above
factors, most batteries fall into an area somewhere between flooded and perfectly
recombinant. Most starved-electrolyte systems have very high recombination efficiencies
at the low current levels typically observed on float, C/100 or less. As the
current levels rise, recombination efficiency drops and oxygen and hydrogen
gassing increase. If excessive currents are experienced, gassing levels become
very high and if this condition is prolonged the cell will dry out. At first,
heavy gassing is the only drawback, but when the weight loss exceeds 5–10% of
the cell fill weight the cell impedance reportedly rises and there is a loss
of discharge capacity.17
However, this is partly offset by the fact that as the cell loses weight the
void volume increases, weight loss per amp-hour of overcharge at a set current
decreases and the rate of gassing diminishes. Unless a cell or battery is heavily
overcharged over a short period of time, drying out is not a common failure
mode for VRLA systems. Batteries will usually fail due to mechanical defects
or leaks, followed by grid corrosion and/or shorting. If none of these cause
failure, then drying out will probably be the failure mode. This is signaled
by rapidly increasing end-of-charge or float currents and if the units are not
removed from service they will self-destruct via thermal runaway.
As mentioned briefly before, hydrogen gas generated at the negative can theoretically
undergo its own recombination reaction at the positive according to the following
process:
|
H2
+ PbO2 + H2SO4
« PbSO4 +
2H2O
|
(1) |
|
|
 |
|
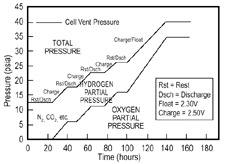
Figure 4. Internal cell gas pressures during cycling and float charging. |
The positive-plate film thickness is relatively small and the diffusion coefficient
of hydrogen is roughly three times that of oxygen, so, if anything, the hydrogen-recombination
efficiency should be greater than that for oxygen. Such a reaction or even direct
combination between H2 and O2
are thermodynamically favored but kinetically hindered. Hydrogen recombination
has been proposed as occurring in VRLA systems 18–20
and has been shown to take place on battery straps to a limited extent. It does
not appear to take place at measurable rates in most commercial battery systems;
diffusion through the plastic cell container is a more likely pathway to relieve
any hydrogen pressure buildup. A further confirmation of this can be seen in
Figure 4, which contains data for hydrogen,
oxygen, and total gas monitoring within a VRLA cell during discharge, over-charge
and rest periods, followed by float charging. Note that, during the roughly
ten-hour rest/discharge periods, the hydrogen partial pressure is slightly dropping
or constant (within the accuracy of these measurements) and, as long as the
total pressure does not reach the venting value, both the hydrogen and total
pressures continue to rise during recharge and float periods. The dotted lines
depict what is likely to be the hydrogen excursions during recharge, where a
pressure increase would only be anticipated at the end when the negative goes
into overcharge (gas measurements were only taken at the beginning and end of
each step).
Given the electrolyte amounts necessary to have an effective level of oxygen
recombination—not flooded and not extremely starved—concurrent hydrogen generation
at the negative according to a mixed-potential model, though minor, is not only
inevitable but probably desirable. Because hydrogen effectively does not recombine
in VRLA cells, its buildup and venting must be acknowledged and accommodated.
Whenever a cell or battery is over-charged, in addition to gases some heat will be generated due to polarization and resistive effects and the heats of reaction for the primary and any secondary chemical processes taking place. The effectiveness of the battery or cell in dissipating this heat is a complex function of the unit’s construction, the over-charge conditions, and the surrounding environment.21 In a flooded vented battery, the main chemical heat sources are the overcharge reactions involving water oxidation at the positive electrode and hydronium ion reduction at the negative, according to Reaction A and Reaction F, respectively. The net reaction is the decomposition of water according to the simplified reaction:
|
H2O «
H2 + 1/202
|
(2) |
The heat of reaction, T D S, for this process is
49 kJ/mole at 20°C and corresponds to roughly 20% of the free energy of reaction
DG. Thus approximately 1/5 of the energy put into
the decomposition process is liberated as heat, since this process is exothermic.16
By comparison, the primary cell charge/discharge reaction,
Discharge
|
Pb + PbO2 + 2HSO4–
+ 2H+ « 2
PbSO4 + 2H2O
|
(3) |
Charge
has a heat reaction of 11.6 kJ/mole, which corresponds to about 3% of the free
energy, being negative during discharge (energy absorbed) and positive on charge
(energy liberated).16
This amount of energy is relatively small and is generated over a comparatively
long period of time; it is usually easily dissipated through radiation and convection.
In a vented cell, the heat generated during overcharge will also be given off
partially by conventional heat transfer to, and then from, the battery surface,
but since more heat is created in a relatively shorter period of time an additional
pathway may be necessary to avoid heat buildup.In vented cells,theoxygen (and
hydrogen) recombination efficiencies are very low and so additional heat dissipation
via gas is realized. The heat capacities of oxygen and hydrogen are substantial
(0.22 and 3.41 cal·g. –1 °C –1
, respectively) resulting in removal of roughly 66% of the energy input, or
over-charge current multiplied by the float or charge voltage, via gassing.16
This is adequate to keep battery temperatures at moderate levels at all but
the most severe overcharge rates. In fact, it is virtually impossible to drive
a flooded lead-acid cell into thermal runaway.
For VRLA cells the situation is quite different. Because there combination process
depolarizes the negative electrode, higher currents will flow at a set float
voltage relative to a flooded analog.16,22
This elevated wattage input is exacerbated by the lower gassing rate, and as
a result, in a typical case only about 5% of the wattage input is dissipated
as heat through gassing.16
In the extreme example of perfect recombination, of course,the conversion efficiency
for electrical energy to heat during overcharge or float is 100%.
The amount of heat generated on over-charge in a VRLA cell is thus 2–3 times
that of a vented cell and only about 1/10 as much heat is dissipated through
gassing. As the recombination efficiency is raised,theratioof heat generated
to heat dissipated through gassing increases rapidly, beginning at a value of
about 1.5 for a flooded system and approaching infinity for an ideal recombinant
cell.
|
|
 |
|
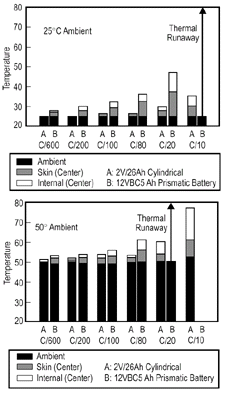
Figure 5. Battery skin and internal temperature as a function of overcharge level and ambient temperature. |
Since heat loss due to gassing is low in VRLA cells and batteries, design factors such as the following are important to optimize heat transfer by radiation and convection:21
This last factor has been effectively addressed by comparisons of 1 ´
4 and 2 ´ 2 cell configurations23,24
and 1 ´ 10 and 2 ´ 5 battery
arrays25 from the standpoint
of heat dissipation via convection and radiation as a function of exposed wall
surface areas. Since the ratio of end cell/ interior cell outer surfaces are
as is greater than 2 in some cases, it was found that temperature variations
within a battery or cell pack can vary greatly, thus possibly affecting cell
failure times for such processes as grid corrosion. In battery arrays, it was
found that the 1 ´ 10 configuration was acceptable
up to a certain size, beyond whic hthe more uniform 2 ´
5 array allowed operation at higher temperatures without the system going into
thermal runaway. Figure 5 illustrates
the same principle in a different way, comparing the thermal characteristics
of cylindrical 25 Ah single cells (with a high surface area/volume ratio) to
those for a prismatic 12V/65 Ah battery. As expected,the latter has less uniform
temperatures and goes into thermal runaway at lower overcharge levels in spite
of the fact that it operates at a lower vent pressure with higher gassing rates
than the cylindrical cells.
All of this suggests that when VRLA batteries are put into closed-cabinet applications
in large arrays, thermal management is acritical consideration, much more so
than for vented lead-acid batteries. Wherever possible, forced convection using
fans and room for spacing between batteries should be implemented in the cabinet
design. Without such precautions, system scan suffer catastrophic failure at
temperatures as low as 37°C.25
Additional measures such as thermo-couple implantation in batteries to allow
for battery temperature-compensated charging will be necessary in certain applications
as usage environments become more and more hostile. Evaluation of the heat generated
by associated electronic equipment will also be a factor in raising the system
temperature baseline off which the battery has to operate. It should be stressed
that environmental temperature/heat dissipation relationships for VRLA batteries
are only roughly linear at lower temperatures; there will be a critical temperature
point where heat generation becomes much closer to exponential.25
Operation in or above this range will have obvious consequences.
1. A. Zierfuss,
German patent 49,423 (1888).
2. O. Jache, U.S. patent 3,172,782
(1965).
3. J. Garche, private communication.
4. T.D. Edison, U.S. patent
1,016,874 (1912).
5. R.F. Nelson (Paper presented
at LABAT ’89, Droujba, Bulgaria, May 1989).
6. P. Ruetschi and J.B. Ockerman,
Electrochem. Technology, 4 (1966), p. 383.
7. J. Thompson and S. Warrell,
Power Sources 9, ed. J. Thompson (London: Academic
Press, 1983), p. 97.
8. B.CulpinandJ.A.Hayman,
Power Sources 11, ed.L.J.Pierce (Basingstoke, Power Sources Committee, 1986),
p. 45.
9. A.J. Salkind, unpublished
data.
10. J.P. Pompon and J. Bouet,
INTELEC ’89 Conf. Proc. (Piscataway, NJ: IEEE,
1989), paper 17.4.
11.J.S.Symanski, B.K.Mahato,
and K.R.Bullock, J.
Electrochem. Soc., 153 (1988), p. 548.
12. J. Ruetschi, J.
Electrochem Soc., 120 (1973), p. 331.
13. K.R. Bullock and M.A. Butler,
J. Electrochem
Soc., 133 (1986), p. 1085.
14. D. Pavlov et al., J.
Electrochem. Soc., 136 (1989), p. 27.
15. Z. Takehara et al., J.
Electrochem. Soc., 136 (1989), p. 620.
16.D.Berndt, INTELEC’88Conf.
Proc. (Piscataway, NJ: IEEE,
1988), pp. 89–95.
17. F.J. Vaccaro and P. Casson,
INTELEC ’87 Conf. Proc. (Piscataway, NJ: IEEE,
1987), pp. 128–131.
18. B.K. Mahato et al.,
J. Electrochem. Soc., 121 (1974), p. 13.
19. M. Maja and N. Penazzi,
J. Power Sources, 25 (1989), p. 229; and part 1 of this series.
20. J. Mrha et al., J. Power
Sources, 27 (1989), p. 91; and references therein.
21. K. Matthes, B. Papp, and
R. Nelson, Power Sources 12, ed. T. Keily (Basingstoke, Power Sources
Committee, 1989), paper no. 1.
22. W.B. Brecht and N.F. O’Leary,
INTELEC ’88 Conf. Proc. (Piscataway, NJ: IEEE,
1988), pp. 35–42.
23.D.Berndt, 5th ERA Battery
Seminar Proc. (ERA Technology,
Ltd., 1989), paper no. 1.4.
24. S. Sasabe et al., Lead
Battery Power for the ‘90’s (London: Lead Development Association, 1988),
paper no. 13.
25. K. Ozaki, ILZRO Third
Int. Lead-Acid Battery Seminar Proc. (ILZRO, 1989), pp. 155–170.
26. B.A. Wittey, INTELEC
’85 Conf. Proc. (Piscataway, NJ: IEEE,
1985), pp. 133–137.
Robert Nelson is with Recombination Technologies LLC.
For more information, contact Robert Nelson, Recombination
Technologies LLC, 909 Santa Fe Drive, Denver, Colorado 80204; telephone (303)
573-7402; fax (303) 573-7403; e-mail nelson7402@aol.com.
Direct questions about this or any other JOM page to jom@tms.org.
| If you would like to comment on the January
2001 issue of JOM,
simply complete the JOM on-line critique form |
|||||
|---|---|---|---|---|---|
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |