 |
|
|---|
 |
|
|---|
|
TABLE OF CONTENTS |
|---|
|
|
|
|
Figure 1.The average consumption of reductants in blast furnaces in France. Adapted from Steiler.7 |
|
|
 |
|
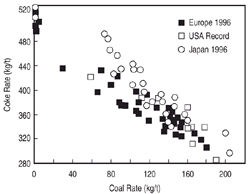
Figure 2. The coke and coal rates by region (average for Europe and Japan; record performance values for the United States). Adapted from Steiler.7 |
|
|
The iron and steel industry has undergone a technological revolution in the last 40 years. In a relatively short time, the North American industry has observed the complete disappearance of basic open hearth processing, as well as the wide spread adoption of continuous casting and the near complete shift of long product production to the electric arc furnace sector. These and other developments have dramatically affected the way steel is made, the price, quality and range of products generated, and changed the basic structure of the industry. The same trends can be observed in other industrialized nations and are reflected in the global industry as well. Competitive forces and market globalization will continue to drive the development and adoption of new iron and steelmaking technologies well into the 21st century. Industry response to specific local and global technology drivers will likely result in both incremental improvements in existing technologies, and in major developments in several key areas including direct iron making and near net shape casting.
The origin of ferrous alloy production can be traced back to
as early as 2000 BC, when writings from ancient China and India made reference
to manmade ferrous metals. By 1350 BC to 1100 BC, the production of ferrous
metals from iron ore had spread to a wide geographic area.1
More than 3,000 years after this early beginning of the Iron Age, modern iron
and steelmakers still use the same carbothermic process discovered by early
ironmakers. However, this industry continues to develop incremental and entirely
novel technology improvements that are more efficient, more productive, and
cheaper than existing processes to produce high quality ferrous alloys with
a wide range of properties and end uses.
Modern ironmaking and steelmaking is extremely intensive in material and energy
usage as well as in capital requirements. The industry is also faced with a
wide range of environmental concerns that are fundamentally related to the high
energy requirements, material usage, and the byproducts associated with producing
more than 725 million tonnes of steel per year worldwide. A highly competitive
steel market, due in part to rapid technological change and accelerating market
globalization, requires the modern steelmaker to be sensitive to customer demands
in terms of product properties, quality, price, and delivery. Although it is
the product of an extraordinary array of high-tech processes, steel is a raw
material in the modern economy and is at the front end of many complex manufacturing
chains. As a result, the steel producer is very sensitive to a dynamic market
with periods of economic boom and slowdown. However, the steel industry is bridled
with high fixed capital costs and processes, which are constrained to high production
rates by efficiencies and economies of scale. A highly competitive world steel
market has, in turn, produced an environment where capital resources are short
and the cost of failed technological ventures dear. Therefore, the risk associated
with being a technology leader is very high. In spite of this, the last 30 years
have shown several times over that the technology of steelmaking can change
rapidly on a global scale. The risk associated with being a technology follower
can also prove to be painful.
The Sloan Steel Industry Study, conducted by Carnegie
Mellon University2,
identified four main technology drivers for the steel industry: high capital
costs, raw materials shortages, environmental concerns, and customer demands.
The American Iron and Steel
Institute (AISI) combined the Carnegie Mellon study and input from a large
number of industry experts to develop a technology roadmap3,
a guide for future R&D efforts in North America. The AISI
Roadmap is intended to focus attention on clear research needs of the industry.
Although some of the technology needs and drivers identified in the AISI roadmap
are specific to the North American industry, many of the identified research
areas are the focus of worldwide research efforts and reflect universal
technology needs for the industry. 4–6
The blast furnace, in various forms, has remained the workhorse
of world-wide virgin iron production for more than 200 years, producing carbon-saturated
“hot metal” for subsequent processing by steelmaking processes. However,
the modern blast furnace has advanced a long way from its earlier ancestors.
Most modern large-capacity blast furnaces represent extremely efficient chemical
reactors, capable of stable operation with an impressive range of reactant feed
materials. The injection of pulverized coal, natural gas, oil, and, in some
cases, recycled plastics to replace a portion of the metallurgical coke used
as the primary reductant and source of chemical energy represents an important
development in the process.
Coke is produced by baking coal in the absence of oxygen to remove the volatile
hydrocarbons contained in coal. The resulting coke is mechanically strong, porous,
and chemically reactive, which are all critical properties for stable blast
furnace operation. In addition to supplying carbon for heat and the reduction
of iron ore, coke must also physically support the burden in the blast furnace
shaft and remain permeable to the hot air blast entering from the bottom. Coke-making
is extremely problematic from an environmental perspective, as many of the hydrocarbons
driven off during the coking process are hazardous. Also, not all types of coal
are suitable for the production of coke. Recently, demand has decreased for
the byproducts from coke-making for secondary processing into chemical products.3
In developed countries, aging coking facilities and tightening environmental
control have made coke-making an economic liability. Therefore, decreasing both
the coke rate and the over-all fuel rate of the blast furnace has been a major
focus of recent developments. Figure 1
shows the evolution of blast furnace consumption of reductants in France in
the last 30 years.
Similar trends can be observed in most developed countries.
However, the relative proportions of coal, natural gas, and oil usage are dependant
on several factors. These factors include local availability, fuel price, and
the capital requirements of the injection equipment. Figure
2 shows the coke and coal consumption rates per ton of hot metal in Europe,
Japan, and the United States. At high coal injection rates, partially combusted
coal char builds up in the area near injector. This can lead to reduced gas
permeability and currently sets the practical limit for coal injection. Extensive
experimentation in the United States and elsewhere has found an optimum combination
of fuels that allows for stable operation at low coke rates. That ideal mix,
per ton of hot metal produced, includes metallurgical coke, 230 kg; nut coke,
40 kg; injected coal, 180 kg; and injected natural gas, 50 kg.
Improvements in process control and reduced refractory wear have increased blast
furnace campaign life significantly, which is critical to the economics of the
process. The current expected lifetime of newly rebuilt furnaces is 20 years
or greater. Improved process control, burden design, regular maintenance, and
reduction of unscheduled shut-downs has had a dramatic effect on the productivity
of blast furnace operations.4–7
Many steel companies have shut down older furnaces while maintaining or increasing
hot-metal production by increasing the productivity of newer furnaces.
In the past 5 to 10 years, there has been a rapid increase in the production
of iron via direct reduction processes. This new production has been dominated
by the gas-based Midrex and Hyl processes, although several new plants based
on other processes have begun production. This additional world wide ironmaking
capacity has primarily served the electric arc furnace industry, providing an
alternative to high quality and expensive scrap as a source of clean, low residual
iron units.
In the last 40 years, the basic open-hearth process has been almost completely
replaced worldwide by various top, bottom, or combination blown basic oxygen
steel making (OSM) processes.1
Since the adoption of basic oxygen steelmaking, continuous incremental improvements
on the various forms of the process have improved the productivity and efficiency
of oxygen steelmaking vessels. Development efforts have included experimentation
with various combination top and bottom blowing configurations, natural gas
shielding of bottom oxygen tuyeres, bottom stirring, top lance design, post
combustion, slag formation control, process monitoring and control, and refractory
design. A recent, important development in oxygen steelmaking has been the adoption
of slag-splashing practices to increase furnace lining campaigns to more than
20,000 heats.3,8
In this practice, the furnace refractory lining is coated with slag between
heats by nitrogen injection into the vessel after tapping of the liquid steel.
Although the solidified slag coating eventually remelts in the subsequent heat
or heats, this practice has effectively extended furnace lining life.
|
|
|
Figure 3. Evolution of steel by process from 1955 to 1996. Adapted from Fruehan.1 (Original source: International Iron and Steel Institute.) |
|
|
 |
|
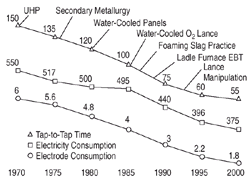
Figure 4. Evolution of EAF performance from 1970 to 2000. (Source: AISI Technology Roadmap.)2 |
|
|
The abandonment of open-hearth steelmaking practices for oxygen
steel-making was accompanied by a parallel widespread departure from ingot casting
and slabbing practices to the continuous casting of steel. The increases in
productivity and yield associated with continuous casting have had a dramatic
effect on the steel industry worldwide. From the mid- to late 1960s to present,
the amount of continuously cast steel as a percentage of total steel production
has risen from essentially 0% to more than 90% in most countries. Most of that
change occurred in the relatively short period from 1970 to 1990.9
Critical research regarding the fundamentals of solidification, defect formation
and modification, fluid flow in the continuous-caster mold and developing steel
shell, refractory interactions, and mold flux design have led to improvements
in the control and reliability of continuous-casting processes. In North America,
this knowledge base was developed through the research efforts of individual
steel companies and through significant contributions by such universities as
Carnegie Mellon University,
the Massachusetts Institute of
Technology (MIT), The University
of British Columbia, The
University of Illinois, and others.
Because oxygen steelmaking processes melt less scrap than open-hearth
steelmaking, the adoption of oxygen steelmaking in developed countries was associated
with a decrease in the price of scrap steel.1
This increase in scrap availability and decrease in price created an opportunity
for growth of scrap-based steel-making. With lower capital costs than an integrated
mill, minimills based upon electric-arc furnace melting (EAF) of scrap were
able to establish a cost advantage for the production of certain steel products.
Figure 3 shows the proportion of crude
steel production by process in the United States over the last 50 years.
The development of ultra-high-powered electric-arc furnaces and reliable billet
and bloom continuous-casting machines provided a low-cost route for the production
of lower quality steel long products, such as reinforcing bar and structural
steels. As a result, integrated steel producers have been completely displaced
from this low-end segment of the steel market in developed countries. This has
allowed integrated producers to focus on the production of high-quality plate
and thin-gauge flat products. The quality of steels produced via EAF is restrained
by the level of metallic residuals such as copper, nickel, and tin, in the scrap
metal charge and dissolved gasses such as hydrogen and nitrogen, which are contained
in the scrap and picked up during processing. At very low levels these contaminants
can significantly degrade the physical properties of many steel grades. However,
continuous improvements in EAF process control and the use of ore-based scrap
substitute materials such as direct reduced iron, hot briquetted iron, and pig
iron to dilute tramp elements in scrap, have significantly increased the product
quality range. Improved chemistry control and the successful implementation
of thin-slab casting by Nucor
has demonstrated that EAF producers can also be competitive in producing quality
flat products as well. The continued expansion of EAF steelmaking for the production
of higher quality steel products is projected to continue.10
However, this expansion will require continued technological development of
the basic process of electric furnace steel-making. More than 40% of steel produced
in the United States is produced by EAF, and that figure is expected to rise
to 50% by 2010.
In the last 30 years, a number of major technical modifications of the electric
arc furnace have dramatically improved the efficiency and productivity of the
process. Up to the present, the primary focus of electric furnace development
has been to increase productivity and energy efficiency by decreasing tap-to-tap
time. Large heat losses occur while the scrap pile or liquid steel is at high
temperature. Greater energy efficiency is achieved when the rate of energy input
is increased and the time at temperature is decreased. As a result, many of
the developments in EAF steelmaking have focused on increasing the net energy
density that the furnace is capable of delivering.1
The development of foamy slag practices, whereby the hot electrode(s) and plasma
arc are enveloped in foamed steelmaking slag, has significantly improved EAF
performance. This practice protects the furnace roof and side walls from radiation
and excessive heating, helps to stabilize the arc, and increases heat transfer
to the steel, thus allowing furnace operators to run at much higher rates of
power input.
Most modern electric furnaces also use a combination of oxy-fuel burners, pulverized
coal injection, and oxygen injection to supplement electrical energy input.
For modern EAF operations, 35% of the energy input is in from chemical energy
sources.3 Recently, additional
chemical energy has been recovered via post-combustion reducing of product gases
by the controlled injection of additional oxygen into the furnace above the
slag. In the most modern furnaces, oxygen injected to combust pulverized coal
injection and carbon charged into the furnace in scrap steel, direct reduced
iron, pig iron, coke or coal can be as high as 40 Nm3/ton. For furnaces with
post-combustion systems, the oxygen usage may be as high as 70 Nm3/ton.1
At these very high rates of oxygen usage, significant additional heat energy
is released by the exothermic oxidation of iron at high temperature. The additional
heat input is gained at the expense of yield, due to the loss of iron as iron
oxide in the slag. As a result, slag chemistry control and yield will become
a focus of future developments in process control. Figure
4 shows the progress of EAF steelmaking in the last 30 years with respect
to several key performance indicators.
The large quantities of hot combustion product gasses generated in the modern
EAF have led to the development of several novel scrap preheating systems, whereby
the heat energy of the exhaust gas is used to preheat scrap prior to melting.
Between 10–30 percent of the energy input into an EAF can leave the furnace
with the hot exhaust gas. Theoretically, 10 kWhr/ton of energy can be saved
for every ~50 8C of preheating of the scrap charge.2
In practice, capture of the heat from furnace exhaust gas has been problematic,
primarily due to emissions control complications. Until recently, relatively
low energy prices have made the economics of scrap preheating marginal, particularly
in cases where the efficient heat transfer could not be achieved. The Fuchs
shaft furnace, the Consteel process, and the Nippon
Steel/Davy-Clecim twin shell electric arc furnace concept are some examples
of scrap preheating systems that are currently in commercial use. Several processes
are under development that allow for continuous preheating, feeding, and melting
of scrap.1
Worldwide, direct-reduction capacity via existing gas-based technologies is likely to increase in order to support the expansion of EAF steelmaking to new, high-quality products. However, the blast furnace is likely to remain the backbone of worldwide iron production for several decades. Current fluidized bed and shaft furnace direct-reduction processes rely on natural gas as the primary reductant and source of heat for the reaction. One exception is the hydrogen-based Circored process. A Circored hot-briquetted iron plant in Trinidad produces reduced iron using byproduct hydrogen from the local petroleum industry. In regions where an abundant and inexpensive source of natural gas (or hydrogen) exists, gas-based direct reduction of iron followed by melting in an EAF can provide a cost-competitive alternative to quality steel products. However, in areas where low cost natural gas is not available, coal-based iron reduction processes will have an advantage. The efficiency and productivity of modern large-capacity blast furnaces will be difficult to surpass. However, high capital requirements make it unlikely that any new blast furnaces will be built in developed countries in the near future. Nevertheless, the shut-down of aging coking operations and older, smaller blast furnaces will force the industry to pursue one or a combination of three options:
|
|
|
Figure 5. Productivity at AK Steel’s Midland No. 3 blast furnace from June 1987 to August 1996. Adapted from Rabold and Hiernaux.18 |
|
|
There are several methods by which a limited supply of hot metal could be stretched
by increasing scrap utilization. Process optimization of current oxygen steelmaking
technologies will result in small improvements in yield by reducing both the
iron content and total volume of slag produced. Scrap preheating and improved
post combustion in conventional oxygen steelmaking vessels could be used to
increase the scrap usage in these processes. Entirely new oxygen steelmaking
furnace designs have been proposed, such as the energy optimizing furnace11,
which makes use of high rates of post combustion, additional fossil fuel additions,
and elaborate scrap preheating to increase scrap melting to as high as 70% during
hot metal refining. Alternatively, direct hot metal addition and increased oxygen
usage in a conventional EAF can dramatically decrease the electrical energy
requirements per tonne of steel. The latter option allows the steelmaker to
produce steel using anywhere from 20% to 100% scrap, producing the entire range
of steel qualities with respect to residual content. Such a hybrid EAF-OSM process
offers a great deal of process flexibility using proven and well-understood
processes.
One limitation of stretching hot metal through significantly increased scrap
utilization is related to the control of residual elements. As mentioned earlier,
the quality of steels that can be produced via conventional EAF steelmaking
is currently limited by control of residual metallic tramp elements in scrap
and dissolved gasses. The increased production of steel long products via EAF
steelmaking has resulted in a general decline in the quality of #2 and heavy
melting scrap steel. If scrap is used in increasing quantities for the production
of all steels, levels of residual elements can be expected to rise in the entire
scrap supply. One solution to this dilemma is an economical process by which
residual tramp elements can be removed from scrap, thus upgrading the scrap
quality. Several processes have been demonstrated on laboratory and pilot scales12–17
that have been successful in removing certain tramp elements. However, in each
case, unfavorable economics have prevented widespread commercial implementation.
One alternative to removing metallic tramp elements is to reduce their deleterious
effects on steel properties. Most metallic residuals reduce steel hot strength
and hot and cold ductility by segregating to and weakening grain boundaries.
Tolerance to such chemical impurities could be improved through the design of
alloys in which these elements were tied up in heterogeneously nucleating second-phase
particles, which might not have the same negative effect on steel properties.
Also, new near net shape casting processes, which will be described in following
sections, may dramatically reduce the overall effect of residual elements for
two reasons. As its name implies, near net shape casting describes solidification
processes by which steel is cast in dimensions near to the specifications of
the final product.
Although hot reduction at some level may still be required for microstructure
control, near net shape casting significantly reduces the dimensional forming
requirements of hot reduction processes and may reduce problems such as hot
tearing. Even more importantly, near net shape casting processes for the production
of thin gauge steel involve much higher rates of heat removal, solidification,
and cooling than conventional casting or thin-slab casting processes. As a result,
microstructural evolution in strip cast materials is fundamentally different
from conventionally processed materials. Macro-segregation in strip-cast steel
is significantly suppressed. Experiments have shown that a wide range of properties
can be achieved from a single steel chemistry entirely through variation of
casting speed and solidification and cooling rates. This has significant implications
for the future of residual element control in steelmaking.
Industry leaders continue to demonstrate the significant potential for increased
productivity of the blast furnace.18
Figure 5 shows the production rate at
AK Steel’s Middletown
No. 3 blast furnace over a ten-year study period.
For blast furnace production to continue into the future even at current levels in the United States and other developed countries, continued progress must be made on reducing the coke rate of furnaces through coal injection. Significant progress has been made in evaluating the benefits of oxygen enrichment of the hot blast. Industrial trials are in progress to evaluate an oxy-coal injection system, which promises to allow for complete combustion at elevated coal injection rates.19 New, environmentally acceptable and economically feasible processes for new or replacement coke production capacity should be evaluated. If less coke is to be used in the blast furnace, the mechanical property requirements of the coke that is used will become more critical to maintaining permeability and stable furnace operation. If antiquated coke production methods cannot produce material of the required strength, exporting the environmental problems of coke production to developing countries with less stringent regulations may become functionally as well as socially unacceptable.
Several new processes for producing hot metal are in various stages of development
around the world. For example, technologies have been developed for the reduction
of iron ore or steel mill waste oxides to produce a solid direct-reduced iron
product. That product could be discharged to a second reactor for melting or
cooled and stored for later use. Several processes based upon the direct reaction
of coal and iron ore in a rotary kiln, such as the SL/RN process, have reached
various stages of development since the 1960s.20
Due to the high gangue and low specific productivity of these processes, they
have not received a great deal of attention for commercial production. Several
processes are currently commercially available that use a rotary hearth furnace
to reduce composite pellets containing both iron-oxide fines from ore or wastes
and carbon from coal, coke, wood char, or mill wastes. Due to the intimate contact
between the carbon and iron oxide in the composite pellets, iron reduction is
very fast at elevated temperatures. The off gasses from the reduction reaction
and/or coal devolatization can be post combusted in the rotary hearth chamber
to provide a significant portion of the heat required for the process. Midrex
is currently marketing a rotary-hearth-based process, Fastmet, for recycling
mill waste oxides.21
Two commercial Fastmet units have been installed at Kobe
Steel and Nippon Steel,
both in Japan. Iron Dynamics, a subsidiary of Steel
Dynamics, currently operates a rotary hearth furnace to produce 85 percent
reduced iron pellets. Those pellets are subsequently melted in a submerged arc
furnace to produce hot metal for use in a nearby Steel
Dynamics EAF shop. The Iron Dynamics rotary hearth-submerged arc process
uses proven technologies to produce liquid iron at a reasonable cost for use
in the EAF.22 However,
the total energy efficiency of this process is not very high as compared with
the blast furnace or other new coal-based technologies.
Several new technologies take advantage of the rapid-reaction kinetics and
high specific productivity of smelting reactors to accomplish at least part
of the reduction of agglomerated, lump, or fine iron ore using coal directly.
Coal devolatization and gasification also occurs in the smelter reactor. Volatile
hydrocarbon compounds make up 10–15 percent of low-volatile coals and 40–45
percent of high-volatile coals.23
In theory, the high-temperature removal and controlled combustion and/or reaction
of these compounds to CO/CO2 and H2/H2O
alleviates some of the environmental problems associated with conventional coke
making.
The Corex process,24
commercialized by Voest Alpine,
combines an iron melter/coal gasifier vessel with a pre-reduction shaft to produce
a liquid product that is very similar to blast furnace hot metal. Coal, oxygen,
and pre-reduced iron are fed into the melter/gasifier to melt the iron and produce
a highly reducing off-gas. The primarily CO-H2
off-gas is then fed through a pre-reduction shaft furnace, where lump and/or
agglomerated ore is reduced to over 90 percent for feeding into the melter/gasifier.
The gas exiting the pre-reduction shaft still has a very high energy content,
which can be used elsewhere in the steel plant or for electric power generation.
Voest Alpine and POSCO
jointly continued to develop the original commercialized process, leading to
several important modifications including the limited direct reduction and smelting
of ore fines 25 . If
the high energy content of the exhaust gas from the reduction shaft is not utilized,
the Corex process requires a relatively high fuel rate as compared with a blast
furnace. Although Corex has a relatively high capital cost 23
, it is so far the only smelting process to be operated on a commercial scale.
The first commercial Corex plant with a capacity of 300,000 tonnes per year
began production in 1989. Other installations are operating, under construction,
or planned in Korea, South Africa, and India.
In the HIsmelt process, iron reduction and coal gasification take place in a
liquid metal bath. The fundamental processes of HIsmelt began with early experiments
in Germany with bottom-blown oxygen steelmaking converters (LD, LD-AC, KMS,
among others) to allow for coal, lime, and/or iron ore injection through the
bottom nozzles.26 Experiments
with combination blown oxygen converters serendipitously discovered that simultaneous
bottom oxygen blowing and soft or low velocity top oxygen blowing resulted in
post combustion of the decarborization product gases in the area above the bath.
High heat transfer rates from the hot post combusted gasses to the metal bath
were achieved via heat transfer to metal droplets ejected into the gas above
the bath, which then fell back into the molten pool. Bottom injection of coal
augmented this post-combustion phenomenon and allowed for significantly increased
scrap melting (100% in the KS process) or smelt reduction of iron ore. Early
experiments by Klöckner
Werke and CRA (now Rio
Tinto) with smelt reduction via simultaneous bottom injection of coke and
ore into a KMS converter indicated that the reduction reaction kinetics were
extremely fast and that the iron reduction, coal gasification, and post combustion
reactions could be predicted and controlled. A small-scale test facility was
built in Germany in 1984 to produce hot metal.
In 1989, CRA and Midrex
formed a joint venture to build a demonstration plant in Western Australia to
further develop the HIsmelt process. Since that time, the process has been significantly
modified, simplified, and improved, allowing for extended continuous operation
and very high specific productivity performance. The extensive pilot scale testing
in Australia resolved many of the technical problems, such as refractory wear,
post-combustion control, and slag-foaming control, which limit the stable operation
of all bath smelting processes.27
One unique feature of HIsmelt is that all reactants are injected through submerged
lances. Pilot scale testing data indicate that this results in much better coal
utilization than with top-charged processes. Like Corex, HIsmelt produces a
hot exhaust gas with significant thermal and chemical energy content, which
can be used for pre-reduction and pre-heating of the iron feed or on-site power
generation. A production-scale demonstration HIsmelt plant producing around
600,000 tonnes per year is planned for Kwinana, Western Australia.
Simultaneous independent development of the direct iron ore smelting (DIOS)
process in Japan 28-30
and the AISI direct steelmaking process in North America 31,23
produced two similar routes to hot metal production. Both processes utilize
a smelting reactor where the primary reactions occur in a deep slag bath as
opposed to in the metal phase as in HIsmelt. Pre-reduced iron ore, coal, and
oxygen are injected into a deep steel-making slag. The coal is devolatilized
and partially combusted to CO. The uncombusted coal char either directly reacts
with iron oxide dissolved in the slag to form iron and carbon monoxide or dissolves
in the iron bath. Dissolved carbon in the metal also reacts with iron oxide
in the slag to form iron and carbon monoxide. Stirring gas injected through
the bottom of the reactor and gas evolved within the slag and at the slag-metal
interface result in foaming of the slag and energetic mixing and intermixing
of the slag and metal phases. Secondary low-velocity oxygen is injected either
above or into the top portion of the slag layer to partially post-combust the
CO and H2 produced by coal devolatilization,
combustion, and iron-oxide reduction reactions. The thick slag layer separates
the iron-carbon melt and char from the oxidizing post-combustion products, providing
a medium for heat transfer. The exiting gas is then used to preheat and pre-reduce
the iron ore feed materials. The DIOS process uses a series of fluidized bed
reactors for preheating and pre-reduction of iron ore fines. The AISI
process uses a Hyl or Midrex
type shaft furnace for pre-reduction and must use primarily lump or agglomerated
ore as its feed material. In these smelter reactors, post combustion provides
approximately 60% of the required energy. However, uncontrolled post combustion
or poor heat-transfer efficiency downward to the bath can cause excessive slag
foaming, damage to the reactor, and generally unstable operation. Precise process
control is required for stable operation. Pilot-scale plants of both the DIOS
and AISI smelter processes
have been built and operated using a variety of feed materials, including low
and high volatile coals, different types of ore, and steel mill waste-oxide
materials. The AISI smelter
has been evaluated as a potential method for the recycling of high iron content
steel mill waste oxides. No commercial production facilities are currentlyplanned
for these two processes.
Several additional combinations of smelting reactors and pre-reduction reactors
are also under consideration. The cyclone converter furnace (CCF), developed
initially by Hoogovens Staal BV, has been considered for use in combination
with the bath smelting reactors described previously.23
In the CCF, iron-ore fines are introduced at the top of the furnace and hot
off-gasses from the smelter reactor enter from the bottom. The feed gas heats
and partially reduces the descending iron ore. Injected oxygen partially combusts
the gas, providing enough heat to melt the iron oxide before it exits the converter.
The intensive mixing of the swirling gasses and iron-ore fines promotes excellent
heat transfer. Hoogovens evaluated the commercial scale-up of a process combining
the CCF with a DIOS type smelter.
The Center for Iron and Steelmaking Research at Carnegie
Mellon University is currently conducting a study, partially sponsored by
the U.S. Department of Energy,
regarding the use of biomass energy sources for hot-metal production.32
The scheme that is currently being evaluated uses a rotary hearth furnace to
heat and partially reduce composite pellets of iron ore fines and wood char.
These pellets are then fed into an AISI
smelter or DIOS-type reactor, where the final reduction and melting occurs.
The off-gas from the smelter would be fed back to the rotary hearth to provide
a portion of the energy requirement of that reactor.
A process which could produce steel or low carbon iron directly and continuously
would be a revolutionary development in ferrous process metallurgy. The AISI
direct steelmaking project evaluated a continuous refining process for the conversion
of hot metal as from a bath smelter to steel.10
The project studied a single zone and two zone reactors. In the two-zone reactor,
hot metal is decarborized to approximately one percent carbon. Final decarborization
occurs in the second reactor. It was found that the slow kinetics of the final
oxidation of carbon from iron at low concentrations resulted in excessive oxidation
of the iron and related control and containment complications. IRSID had developed
a unique design for a continuous steelmaking reactor, which has been tested
on a demonstration scale.10
The IRSID continuous-steelmaking process decarborizes hot metal in a slag-metal
emulsion, which results when oxygen is impinged upon the entering hot metal.
Due to the violent stirring and large metal droplet surface area present in
the emulsion, mass transfer rates in the IRSID reactor are predicted to be 3.3
to 5 times higher as compared with a BOF. However, the violent nature of this
reactor may also result in rapid refractory wearand containment problems.
The IFCON process, developed by ISCOR
in South Africa, is capable of producing steel directly from coal and iron ore.1
In this process, coal and ore are added continuously to a channel type induction
furnace containing a slag-metal bath. Electrical energy is supplied by the induction
furnace for heating and stirring the bath. Oxygen is added for post combustion
of hydrogen and carbon monoxide released from the iron reduction reaction and
the coal. Although the claims of this process are truly revolutionary, few details
regarding the process or test results have been reportedpublicly.
Because the new iron and steelmaking technologies described previously share
some common attributes, they also share some common technical challenges.33
For example, technologies that use coal directly will have to deal with higher
levels of sulfur than the blast furnace. Coal contains both volatile organic
sulfur and mineral sulfur as FeS. During coke making and initial coal pyrolisis
in smelting reactors, the organic sulfur is driven off primarily as H2S.
In the blast furnace and smelter reactors, mineral FeS dissolves in the slag
and a portion is transferred to the metal. In coke making, the volatile organic
sulfur is captured and processed. During smelting, the H2S
will exit with the exhaust gas, where it will likely react with CaO and FeO
dusts to form CaS and FeS. Subsequent dust recycling will result in dissolution
of these phases in the slag and eventual transfer to the metal. In addition,
higher FeO levels in bath smelting slags as compared with the blast furnace
will also promote higher sulfur transfer from the slag to the metal phase. As
a result, highly efficient hot metal desulfurization practices will be necessary
when using bath smelter metal to produce high quality steel products. Fundamental
research at Carnegie Mellon University regarding the kinetics of sulfur transfer
resulted in the development of a kinetic model that can be used to predict and
control sulfur in smelter metal.34
New iron-making processes that use coal directly will generate a large volume
of carbon monoxide, hydrogen, and hydrocarbons, which must be utilized to avoid
condensation of complex and hazardous hydrocarbon compounds and improve the
energy efficiency of the processes. Most new technologies under investigation
use some form of post combustion to supply a large amount of the energy required
for the endothermic reduction of iron oxide. The post-combustion degree (PC)
can be measured as the proportion of the combustion products and reactants in
the off gas of the reactor.
| PC = |
CO2 + H2O
CO2 + H2O
+ CO + H2
|
In conventional reactors such as the BOF and EAF, additional oxygen injected at low velocity above the slag-metal bath combusts CO and H2 to CO2 and H2O, releasing a large amount of heat energy. However, the oxidizing product gasses must be shielded from the metallic iron and carbon in the metal and char to prevent depost-combustion reactions, namely:
CO2 + C ®
2CO
CO2 + Fe ®
FeO + CO
H2O + C ® H2 + CO2
H2O + Fe ®
FeO + H2
These reverse reactions can make it very difficult to attain high degrees of
post combustion when the hot metal and post combustion gasses are in intimate
contact. At the same time, the intended goal of post combustion is to transfer
the heat generated by the combustion reactions downward to the slag and metal.
Poor heat-transfer efficiency (HTE) to the slag and metal can result in an unacceptable
increase in thermal load imposed on the vessel roof and sidewalls and the gas
handling system. In bath-smelting reactors, the iron reduction and coal pyrolisis
reactions are endothermic, thus heat transfer to the site of the reactions can
be a rate-limiting factor for the process. In addition, special care must be
taken when designing the gas handling system for bath-smelting reactors. The
large volumes of hot gas produced and high particulate content of the gas can
easily overwhelm an insufficient system. The HIsmelt process uses preheated
air instead of oxygen for post combustion.26
The post-combustion products are diluted by the nitrogen content of the air,
allowing for simultaneous high post-combustion degrees and high heat-transfer
efficiency. This also increases the total volume of gas exiting the vessel.
In the DIOS and AISI smelter reactors, submerged post combustion, or post combustion
in the top portion of the deep slag layer using submerged oxygen injection,
was investigated.33 Combustion
within the slag layer should result in high heat-transfer efficiencies from
the post combustion reactions to the slag and, subsequently, to the metal. If
the post combustion is limited to only the top portion if the slag bath, the
metal droplets and char should be shielded from the oxidizing product gasses
by the thick slag layer. Both the potential benefits and limitations of post
combustion were studied extensively for each of the bath-smelting processes
described above. Experience gained from the continued development of post combustion
systems for conventional reactors will aid in the design of future post-combustion
systems for bath-smelting reactors.
Steelmaking slags tend to foam when gas is passed through them. The properties
of slag (i.e., high viscosity and high surface tension) are such that the kinetics
of bubble film rupture are relatively slow, and thus, bubbles tend to form stable
rafts. Slag foaming is a feature of most conventional steelmaking processes.
In the modern EAF, maintaining a stable foamed slag to submerge the arc is critical
for high productivity operation. In the BOF, controlling slag foaming is important
for the prevention of slag slopping from the vessel during processing. In the
deep slag-melting processes such as DIOS and the AISI
smelter, a highly stirred, foamy slag forms the medium in which the iron reduction
and coal pyrolisis reactions take place. A large amount of gas is passed through
or generated within the thick slag layer due to bottom stirring gas injection
and the in situ production of carbon monoxide and hydrogen from the reduction,
pyrolisis, and combustion reactions. The control of slag foaming is critical
to the stable operation of the processes. The fundamentals of slag foaming were
studied extensively on a laboratory scale 35,36
and also in the actual pilot-scale reactors 28-31
.
It was found that by maintaining a certain amount of char in the smelter slag
and/or operating at an elevated pressure-slag foaming could be controlled to
an acceptable degree.
Process monitoring and control are critical for stable operation of smelting
reactors. The development or implementation of inexpensive, durable sensors
capable of continuous real time measurement of key process parameters, such
as slag height, post combustion degree, temperature, reactant feed rates, and
possibly slag and/or metal chemistry, are critical to the commercial success
of a smelting process. Significant recent advancements have been made in the
development of such technologies for use with conventional iron and steel-making
processes.37
All the bath-smelting reactors described previously are vigorously stirred reactors
intended for continuous operation possibly at elevated pressures. Refractory
wear is a significant concern for bath smelting processes and all conventional
iron- and steelmaking processes. The use of water-cooled panels can alleviate
this problem, but also reduces the energy efficiency of the process. The development
of erosion-resistant refractories and hearth designs, combined with water-cooled
panels in problem areas, has significantly extended blast furnace hearth life.
Reliable containment is possible in similar solutions for smelting reactors,
but continued refractory development will make these and conventional processes
more reliable.
Fundamental research has played a critical role in the development of conventional
and emerging technologies for iron and steelmaking.38
Many of the findings and contributions of fundamental research toward these
specific technologies have been discussed previously. In the case of each process
described, simultaneous fundamental reactions and physical phenomena were investigated
along with the applied development of the processes themselves. In North America,
an extensive fundamental research program conducted by participating companies
and universities accompanied pilot-scale testing of the AISI
bath-smelting process. The fundamental physical chemistry of smelting reactions
were investigated at Carnegie Mellon
University and by J.F. Elliot at MIT.39
These studies were critical in determining the mechanisms and fundamental rates
of the important reactions occurring during bath smelting.40,41
The previously mentioned studies of slag foaming and hot metal desulfurization
were also conducted at Carnegie
Mellon. In addition, fluid-flow and heat-transfer studies were conducted
at McGill and McMaster
Universities.42,43,44
All of these fundamental studies contributed to a process model for the bath
smelter, which was both accurate and founded on first principals. Continued
fundamental investigation of the kinetics of slag-metal reactions and modeling
of post-combustion processes will aid in understanding and controlling conventional
and future iron- and steelmaking processes.
Research at Boston University
(BU) is in progress to simultaneously examine the electrochemical nature of
slag-metal reactions relevant to iron and steelmaking, and to re-examine and
better define the interactions affecting liquid phase mass-transfer kinetics.
This study has shown that the kinetics of iron-oxide reduction from slags can
be significantly enhanced by short circuiting the electro-chemical reaction.45
The electrochemical nature of slag-metal reactions implies that the half-cell
reactions can be separated to physically distant but electrically connected
sites. Experiments at MIT and
BU have shown that imposing
a plasma field on a liquid iron-slag cell can increase the reaction sites available
for the ferrous-ion-reduction reaction. Therefore, the kinetics of iron-oxide
reduction from slags can be significantly increased under conditions where the
mass transfer of ferrous ions is normally the rate-limiting step. This research
has strong implications for control of slag metal reactions in ultra-high-powered
direct current electric-arc furnaces, which operate with a strong applied electric
field across the slag layer.
Also studied were the effects of pressure on the kinetics of iron-oxide reduction
from slags via an iron-carbon bath. This and previous studies 41
showed a strong correlation between the rate of carbon monoxide evolution generated
by the reaction and the mass-transfer coefficient for ferrous ions in the slag.
This correlation results from the stirring effect of the gas generation at the
slag-metal interface and subsequent rise of bubbles through the slag layer.
Because the ambient pressure at which the reaction occurs has a strong effect
on the nucleation and volume of the carbon monoxide that is generated, it is
not surprising that the liquid mass-transfer coefficient is an inverse function
of the pressure. Experiments at BU
have shown that the kinetics of iron-oxide reduction from slag by an iron carbon
bath increase by a factor of 5 to 10 when the reaction is conducted at reduced
pressure. 46 A correlation
based on dimensionless groups is being developed to predict the mass-transfer
coefficient for ferrous ion transport as a function of ambient pressure. Such
a correlation would be a valuable tool in improving existing models for smelter-type
reactors, which are operated at various pressures in order to control slag foaming.
In addition, the combined results of the experiments with an imposed electric
field and those conducted at reduced pressure suggest that the kinetics of iron-oxide
reduction can be significantly enhanced. A reactor that was able to take advantage
of these two effects would have a very high specific productivity (tonnes per
time per unit volume).
Nearly all the current and future developments discussed so far have been related
to alternative routes to liquid steel. These past, present, and future changes
are mirrored by developments in steel casting technology. Beginning 35 years
ago, the revolutionary industry wide implementation of continuous casting changed
the way liquid steel was solidified for subsequent mechanical shaping to finished
products. Continuous casting brought global changes to steel mill productivity,
the chemistries of steels produced, the secondary processing that was required,
and the properties of the final products. Roughly 15 years ago, successful implementation
of thin slab casting at Nucor shook the established balance of the steel products
produced by minimills and integrated steel-makers. The next wave in the casting
revolution is close at hand. Near net-shape casting is the solidification of
steel to geometry near the dimensions of the final product. Near net-shape casting
processes reduce hot reduction or forming requirements, thus decreasing capital
costs, energy requirements, and delivery time for steel products. Thin slab
casting for flat products and dog bone shaped casters for structural steel members
such as I or H beams represent a step closer toward near-net-shape casting processes.
Sheet steel is an important segment of worldwide steel production in both tonnage
and value. Quality flat products for such applications as exposed automotive
body components represent high value products. Presently, integrated steel mills
using conventional thick slab continuous-casting machines produce the majority
of these high end products. Conventional slab casters produce a continuous
strand of steel approximately 250 mm thick and of various widths. The continuous
strand is cut into slabs that are cooled and stored or shipped directly to
the hot rolling mill. At the hot rolling mill, the slab is reheated and rolled
in a series of strands to a thickness of a few millimeters and coiled for subsequent
cold rolling, heat treating, surface cleaning, and coating.
Thin slab continuous-casting machines produce a slab approximately 50-60 mm
thick. This significantly reduces the amount of hot rolling required to produce
thin sheet, thus allowing for in-line hot rolling of steel as it comes off the
caster.47 However, because
the slab produced by thin slab casting machines is 1/5 the thickness of that
produced by conventional thick slab casting, the thin slab caster must cast
approximately five times faster to match the productivity of the conventional
caster. Unfortunately, there are limits to the casting speed that can be safely
and reliably achieved in thick or thin slab casting machines.48
The residence time of the steel in the caster mold must be long enough to allow
the solidification of a steel shell strong enough to contain the liquid metal
core of the slab as it exits the bottom of the mold. To increase the casting
speed, the length of the mold must be increased proportionately such that this
minimum residence time is maintained. However, as the length of the mold increases,
the friction force between the mold and the moving steel slab also increases,
requiring a greater force to pull the steel out of the mold. The maximum casting
speed is reached when the length of the mold results in a friction force and
associated pulling force that exceeds the hot tensile strength of the steel
shell of the solidifying steel slab. Schwerdtfeger calculated a maximum safe
casting speed of five meters per minute and ten meters per minute for ferritic
and austenitic steels, respectively, assuming that dry, solid/ solid friction
will occur between the caster mold and the solidifying steel.48
In modern continuous-casting practice, complex low melting temperature mold
slags or fluxes are used to provide lubrication between the mold and solidifying
steel shell, as well as to improve heat transfer. Ideally, a continuous liquid
film of mold flux would eliminate dry friction between the solidifying steel
and the mold, in which case there would be no limit to casting speed. However,
in practice, the performance of mold fluxes as lubricants is not as reliable
as would be desired for very high casting speeds. Advances in the fundamental
understanding of the behavior of continuous-casting fluxes will aid in improved
design and more reliable performance of these important materials.49-50
Two new near-net-shape casting technologies are under development for flat steel
products. Henry
Bessemer first envisioned twin-roll hot-strip casting in the mid-1800s.
In its simplest form, the process involves pouring liquid steel between two
large counter-rotating water-cooled rolls. As the rolls rotate, the liquid steel
begins to solidify a thin shell against the cold rolls, and at the “kissing
point” of the two rolls, the two solid shells are pressed together and
exit the bottom of the mold as a single strip of steel. This process is intended
to produce steel strip with a thickness of approximately 2 mm or less. Therefore,
in order to match the productivity of a conventional thick slab caster operating
at a casting speed of roughly 1 to 3 meters per minute, a twin roll strip caster
would have to produce 2 mm strip at speeds on the order of 200 meters per minute
or higher. Heat removal from the steel by the cold rolls is limited by the heat-transfer
coefficient between the two materials. Therefore, the casting speed can only
be increased by decreasing the gap between the two rolls and the thickness of
the steel sheet produced or by increasing the diameter of the rolls. Several
steel companies worldwide have maintained active research programs into the
fundamental and operational aspects of twin-roll strip casting in the past decade.51
Due to the high heat fluxes required for twin-roll strip casting, the fundamental
aspects of heat transfer and solidification are different from conventional
casting processes. Extensive fundamental research programs have been necessary
to develop this technology.52,53
A twin-roll casting process for stainless steel strip production has been developed
in Japan. Through its extensive, decade-long research effort code named “Project
M,” BHP has developed a strip-casting process capable of producing low
carbon strip steel with excellent dimensional tolerance and surface quality.54
Material produced by the BHP commercial-scale demonstration facility in Port
Kembla, Australia has been evaluated and found to exhibit truly unique properties.
Recently, Nucor, BHP, and
IHI formed a joint venture to install a commercial-scale twin-roll strip-casting
facility at a Nucor facility
in the United States. Construction of this facility has been completed and start-up
is scheduled for later this year. Results regarding the performance of this
facility over an extended period are eagerly awaited.
The high rate of heat transfer and rapid solidification that occurs in the twin-roll
strip caster produces a microstructure unlike any other steel-casting process.55,56
Tests with strip cast steel samples indicate that a wide range of mechanical
properties can be obtained from a single steel chemistry through variations
in the casting speed (solidification rate) and subsequent hot and cold rolling
and heat treating. Macro-segregation of impurities in strip cast steel is significantly
suppressed. Therefore, the rapid solidification rate of strip cast steel may
also lead to a greater tolerance for impurity elements, which degrade the properties
of conventionally processed steel products. Precise control of the mechanical
properties of steel products without the stringent steel chemistry control that
is currently required would have a revolutionary effect on the steel industry.
A second technology for hot strip steel production has received less attention
than twin-roll casting. The single-belt casting process,48,57
developed at the Institut
für Allgemeine Metallurgie of Technische Universität Clausthal
in Germany, possesses some unique attributes and capabilities. In this process,
liquid steel is distributed evenly onto a water-cooled steel belt via a dispenser
and air knife. The steel belt is cooled from the bottom and held in place by
a vacuum so that little warpage occurs during solidification. The resultant
steel strip, approximately 10 mm thick, is then fed from cooling belt into an
in-line hot-rolling stand for subsequent hot reduction. Critics of near-net-shape
casting processes claim that internal porosity remaining after solidification
can only be healed by subsequent reduction by a factor of 70-80 percent. In-line
hot rolling of the 1 cm strip from the single belt caster to a thickness of
1 to 2 mm is adequate for this purpose. Higher casting speeds are possible with
single-belt casting by extending the length of the cooling belt and table. This
feature offers greater process flexibility with more favorable economics as
compared to the varying diameter of the large copper alloy rolls of twin roll
casting processes. The ability to cast a thicker strand allows for greater flexibility
in subsequent thermo-mechanical processing of the steel for microstructural
development and control of mechanical properties. One of the primary difficulties
of the process is related to controlling the flow of steel onto the belt and
related thickness variations of the free surface of the steel strand.57
Both laboratory-scale and pilot-scale single-belt casters have been operated
at Clausthal
Technical University. Most of the technical problems associated with operation
of the pilot scale caster have been solved. However, problems associated the
scale up of the process to continuously cast wider strands over the course of
many heats can only be addressed when the process is implemented on a production
scale.
New and conventional casting technologies will continue to strive toward the
production of cleaner steels, free from oxide-inclusion defects. In conventional
casting processes, the significant mechanical deformation required to reduce
the steel to the product dimensions tends to break up inclusions, thus reducing
their effects on mechanical properties. In near-net-shape casting processes,
little mechanical deformation will be required. Therefore, inclusion control
may be critical to the success of these processes. Basic research regarding
the fundamental mechanisms of inclusion formation and separation will play an
important role in the continued improvement of steel properties and performance
and the success of near-net-shape casting processes. Conventional mechanical
filters similar to those used in aluminum processing have not been successful
in removing inclusions from steel due to rapid degradation at steelmaking temperatures.
However, an innovative chemical/mechanical filter is under development at the
University of Alabama, which
offers great promise.58
The steel industry is often regarded as a fully matured industry, using proven processes with only incremental technological developments. However, the past 30 years have witnessed several dramatic technological developments, which have changed the organizational structure, productivity, efficiency, and product properties of the steel industry worldwide. Several exciting new technologies for the production of steel have advanced to a fairly developed stage and will likely be implemented on a production scale sometime in the future. The new technologies will develop in parallel with continued incremental improvements in reliability and energy and materials efficiency of conventional processes. However, the extremely competitive marketplace of the modern steel industry has resulted in an environment where capital resources for research and development are limited and tolerance for failed technology concepts is very low. Therefore, the continued improvement of conventional processes and development and implementation of new technologies will depend heavily upon the determination, creativity, and resourcefulness of the men and women who are up to the challenge.
The authors would like to acknowledge the financial support for research in iron and steelmaking of the Sloan Foundation, the National Science Foundation and the U.S. Department of Energy at Carnegie Mellon University, and the National Science Foundation (Division of Design, Manufacture and Industrial Innovation) at Boston University.
1. R.J. Fruehan,
ed., The Making Shaping and Treating of Steel, 11th Edition—Steelmaking
and Refining Volume (Pittsburgh,PA: AISE,
1998), p. 103.
2. R.J. Fruehan et al., The
Future of Steelmaking and its Technologies (Idaho Falls, ID: Idaho National
Research Laboratory,1995).
3. Steel Industry Technology
Roadmap (Washington, D.C.: American
Iron and Steel Institute, February 1998); www.steel.org/mt/roadmap/roadmap.htm.
4. G. Kolb and H.B. Lüngen,
1998 ICSTI/Ironmaking Conf. Proc. (Warrendale, PA: ISS,
1998), pp. 75–83.
5. Y. Okuno and M. Nose, in
Ref. 4, pp. 67–74.
6. R.J. Fruehan, in Ref.
4, pp. 59–66.
7. Jean-Marc Steiler, in Ref.
4, pp. 161–173.
8. Charles J. Messina and John
R. Paules, 1996 Steelmaking Conf. Proc. ((Warrendale, PA: ISS,
1996).
9. Manfred M. Wolf, 1995
Electric Furnace Conf. Proc. (Warrendale, PA: ISS,
1995), pp. 259–280.
10.
R.J. Fruehan, Scandinavian
J. Metallurgy, 28 (1999), pp. 77–85.
11. R.J. Fruehan and C.L. Nassaralla,
ISS Transactions, Iron
and Steelmaker (August 1998), pp. 59–68.
12. I. Jimbo, M.S. Sulsky,
and R.J.Fruehan, Iron
and Steelmaker (August 1988), pp. 20–23.
13. R.J. Fruehan and A.W. Cramb,
48th Electric Furnace Conf. Proc. (1990), pp. 11–14.
14. R.J. Fruehan et al., Met.
Trans. B, 25B (2) (1994), pp. 306–308.
15. T. Nagasaka, M. Hino, and
S. Banya, 15th Process Technol. Conf. Proc. (Warrendale, PA: ISS,
1997), pp. 41–49.
16. M. Iwase, K. Tokinori,
and H. Ohshita, Iron
and Steelmaker (July 1993), pp. 61–66.
17. M. Iwase, K. Tokinori,
and H. Ohshita, Scandinavian
J.Metallurgy (February 1998), pp. 24–30.
18. C. Rabold and R Hiernaux,
in Ref. 4, pp. 175–185.
19. Michael Riley, DOE-OIT
Sponsored Research "Hot Oxygen (2001); www.oit.doe.gov/factsheets/#steel).
20. R. Panigrahi and I. Dasgupta,
"Direct Reduction Processes," (Warrendale, PA: ISS,
1999), pp. 99–119.
21. www.midrex.com/iron/fast.asp.
22. L.J. Lehtinen, J. Hansen,
and N. Rokop, AISE Proc. of the1999 Annual Convention and Iron and Steel
Exposition (Washington, D.C.: AISI,
1999).
23. R.J. Fruehan, Direct
Reduced Iron—Technology and Economics of Production and Use (Warrendale,
PA: ISS, 1999), pp. 163–171.
24. Michael Lemperle, Proc.
Savard/Lee Int. Symp. on Bath Smelting (Warrendale, PA: TMS,
1992), pp. 159–176.
25. J.L. Schenk et al.,
Iron and
Steelmaker (July 1998), pp. 39–44.
26. K. Brotzmann, Proc.
Savard/Lee Int. Symp. on Bath Smelting (Warrendale, PA: TMS,
1992), pp. 29–38.
27. R.J. Dry, C.P. Bates, and
D.P. Price, 1999 Ironmaking Conf. Proc. (Warrendale, PA: ISS,
1999); reprinted at www.hismelt.com.au.
28. M. Ishikawa, 1996 Ironmaking
Conf. Proc. (Warrendale, PA: ISS,
1996), pp. 415–421.
29. K. Saito, in Ref.
26, pp. 579–590.
30. T. Kitagawa et al., in
Ref. 26, pp. 611–622.
31. E. Aukrust., in Ref.
26, pp. 591–610.
32. R.J. Fruehan., in Ref.
26, pp. 233–248.
33. O. Fortini et al., DOE-OIT
Sponsored Research (2001); www.oit.doe.gov/factsheets/#steel.
34. P. Iwamasa and R.J. Fruehan,
Met. Trans. B,
28B (1) (1997), pp. 47–57.
35. Y. Zhang and R.J. Fruehan,
Met. Trans. B,
26B (1995), pp. 803–812.
36. Y. Zhang and R.J. Fruehan,
Met. Trans. B,
26B (1995), pp. 801–819.
37. Sandia National Laboratory,
DOE-OIT Sponsored Research (2001); http://www.oit.doe.gov/factsheets/#steel.
38. R.J. Fruehan, Iron
and Steelmaker (July 1996), pp. 25–34.
39. G.G. Krishna Murthy and
J.F. Elliot, Ironmaking and Steelmaking, 22 (5) (1995), pp. 405–415.
40. D.-J. Min and R.J. Fruehan,
Met Trans. B,
23B (1992), pp. 29–37.
41. B. Sarma, A.W. Cramb, and
R.J. Fruehan, Met Trans.
B, 27B (1996), pp. 717–730.
42. H. Gou et al., Iron
Steelmaker (January 1995), pp. 47–54.
43. H. Gou, G.A. Irons, and
W-K. Lu, Met. Trans.
B, 27B (2) (1996), pp. 195–201.
44. Z. Lin and R. Guthrie,
Iron & Steelmaker
(May 1995), pp. 67–73.
45. D. Woolley and U. Pal,
Met. Trans. B
(October 1999), pp. 877–899.
46. S. MacDonald and U. Pal,
Proc. of the 2001 NSF Design, Service and Manufacturing Grantees and Research
Conf. (Dearborn, MI: SME,
2001).
47. K. Schwerdtfeger, Iron
and Steelmaker (April 1995), pp. 25–31.
48. K. Schwerdtfeger, ISIJ
Int., 38 (8) (1998), pp. 852–861.
49. A.W. Cramb et al., Steelmaking
Conf. Proc. (Warrendale, PA: ISS,
1995), pp. 655–667.
50. C. Orrling et al., Iron
and Steelmaker (January 2000), pp. 53–63.
51. A.W. Cramb, Near-Net-Shape
Casting in the Minimills (Vancouver, Canada: Canadian
Institute of Mining, Metallurgy and Petroleum, 1995), pp. 355–372.
52. W. Blejde, R. Mahapatra,
and J. Fukase, Iron and Steelmaker (February 2001), pp. 43–48.
53. L. Strezov and J. Herbertson,
ISIJ Int., 38 (9) (1998), pp. 959–966.
54. W. Blejde, R. Mahapatra,
and J. Fukase, Iron
and Steelmaker (April 2000), pp. 29–33.
55. K. Kumari et al., J.J.
Jonas Symposium on Thermomechanical Processing of Steel as held at the 39th
Annual Conf. of Metallurgists of CIM (Vancouver, Canada: Canadian
CIM, 2000), p. 629.
56. K. Mukunthan et al., The
Brimacombe Memorial Symp. (2000), pp. 421–437.
57. K. Schwerdtfeger et al.,
ISIJ Int., 40 (8) (2000), pp. 756–764.
58. R. Bradt and M.A.R. Sharif,
DOE-OIT Sponsored Research (2001); www.oit.doe.govfactsheets/#steel.
Elizabeth A. Holm is a primcipal member of the technical staff of the Materials and Process Modeling Department of Sandia National Laboratories. Corbett C. Battaile is also with Sandia National Laboratories.
For more information contact C.P. Manning, Boston University, Manufacturing Engineering Department, 15 St. Mary’s Street, Brookline, MA 02446; (617) 353-2842; fax: (617) 353-5548.
Direct questions about this or any other JOM page to jom@tms.org.
| If you would like to comment on the October 2001 issue of JOM, simply complete the JOM on-line critique form | |||||
|---|---|---|---|---|---|
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |