 |
47 (11) (1995), pp. 20-25. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
 |
47 (11) (1995), pp. 20-25. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
Editor's Note: For more information on George J. Binczewski and his research, see the article "LMD Member Pioneers Research into the Point of the Washington Monument" in this issue's edition of Division News.
Author's Note: When dealing with historical numbers involving monetary units, it is well to give the reader a perspective about the relationship to current times; it is insufficient to use the financial term inflation adjusted. The author prefers to compare the 1884 price of aluminum of $1 per ounce ($16 per pound) to the fact that in 1884 the wage of a laborer on the Washington Monument was $1 per day, and the workday was typically 10 hours or greater in length. Thus, the cost of one ounce of aluminum was equivalent to a full day's work. The highest skilled craftsman on the monument project was paid $2 per day.
Just as the Washington Monument is an important symbol for the people of the United States, so also is the aluminum pyramid cap at its apex a symbol for the aluminum industry. Indeed, it was accorded this high accolade 50 years after its placement by Edgar H. Dix, then chief metallurgist of the Aluminum Company of America (now Alcoa) when he declared "the crown jewel of the aluminum industry is the cap of the Washington Monument." He made the statement as one of a very small group of experts asked to inspect the condition of the pyramid after 50 years of exposure during the first refurbishing of the monument in 1934. Dix described his overall investigative appraisal in a published article.1
The remainder of this article concentrates on the little known and seemingly insignificant events associated with the choice of aluminum for the apex point, which marked the culmination of the monument's construction in December 1884. For a brief overview of the aluminum industry at the time, see the sidebar on page 21.
Aluminum was not the first choice for the pyramid, nor did the choice involve any material design evaluation, extensive tests, or lengthy comparative competition among available materials. Instead, aluminum was selected as an alternative material during discussions between the engineer in charge of the project and William Frishmuth, the only U.S. aluminum producer at that time.
The engineer in charge of completing the Washington Monument was Colonel Thomas Lincoln Casey, Corps of Engineers, United States Army. He was familiar with William Frishmuth since Frishmuth's foundry in Philadelphia had previously done some plating work for the monument. As the completion of the construction was nearing, Casey sent a request to Frishmuth asking if he could make a metal pyramid that was to serve as the lightning rod. Copper, bronze, or brass, plated with platinum, were the preferred materials. The actual letter is shown in Figure 1.
Figure 1. A copy of the original letter requesting a cast metal pyramid for the Washington Monument point.
Casey, in accepting Frishmuth's proposal, admonished him that "It is desired that the cost be kept if possible, within the estimate which you submitted, but should that cost be necessarily and unavoidably exceeded in producing a perfect piece of workmanship, the account shall be submitted setting forth that fact."
Frishmuth quickly found that he could not use an ordinary sand mold for the casting and had to get an iron mold made. On November 12, 1884, within two weeks of Casey's request, he sent the often-quoted telegram to Casey proclaiming his success:
"After hard work and disappointments, I have just cast a perfect pyramide of pure aluminum made of South Carolina Corundum. Great honor to you, the Monument and whole people of North America & a little to myself lent. Cost of pyramide more than calculated. However will state the facts. When do you want the pyramide. Would like to exhibit for 2 days in this city before express same to you. Answer by messenger. "On November 29, 1884, after receiving the pyramid from Frishmuth, Casey sent a letter to Frishmuth stating, "the point is received and is acceptable in every way."7
When Frishmuth submitted his bill for the aluminum pyramid it was for $256.10. Keeping in mind that Frishmuth originally quoted $75 for the aluminum pyramid, it is understandable that Casey was quite upset. The very day the invoice arrived, Casey cut a military order dispatching his assistant, Captain Davis, to go to Philadelphia to investigate the matter. After Davis concluded his audit, a final price of $225 was agreed upon between Casey and Frishmuth.8,9
The capping ceremony of December 6, 1884, and the formal dedication of the monument on February 21, 1885, were given front-page publicity in the nation's newspapers and the aluminum point or apex was creditably described. Hundreds of thousands, perhaps millions, of people who had never before even heard about aluminum now knew what it was.
The pronouncement by Winckler2 cited in the sidebar on the aluminum industry that "nobody knows how to cast it" (aluminum) was made only five years earlier in Europe, where almost all of the world aluminum activity had been occurring. Of course, Frishmuth had his own experience with the metal, but he was attempting to make a larger casting than anyone had previously done. (The sidebar below details Frishmuth's achievements.) He allowed himself the option of making the pyramid in bronze if his aluminum effort was not successful. Further compounding his difficulty were the demands made in Casey's subsequent agreement letter of Oct. 29, 1884, "to secure a solid homogeneous casting," and "a perfect casting of pure aluminum; producing a perfect piece of cast metal quality."Frishmuth knew that the pyramid's sides, after polishing, would be meticulously scrutinized and that any blemish, sand hole, inclusion, or porosity would be unacceptable.
It should be remembered that during that period there was virtually no knowledge of the dissolved gas in aluminum and its manifestation as porosity in the finished casting. Sixty years later, Eastwood10 cited the case of cast aluminum automobile steering wheels made in the 1915 period that "were polished to a high luster in which the condition of pinhole porosity stuck out like a sore thumb." Frishmuth's casting 30 years earlier avoided this. What did he know in preparing the metal?
There were no filtering or degassing procedures for casting aluminum in 1884. Melting, solidifying, and remelting was the primary means of improving final cast metal quality. The polishing methods for the large surfaces of the pyramid, together with the relatively soft aluminum metal, would have exacerbated any cast surface imperfection (e.g., the familiar comet tail encountered today by people inexperienced in polishing small aluminum metallographic specimens). Finally, the inscriptions engraved on the pyramid's sides would have been adversely affected by any surface imperfection and undoubtedly the meticulous Casey would not have tolerated or accepted this.
The casting's size was only one part of Frishmuth's achievement. The quality of it was quite another.
Two sources reporting the composition of the pyramid indicate 1.70% Fe, 0.55% Si, and 97.75% Al, and 1.90% Fe, 0.61% Si, and 97.49% Al. Interestingly, during the 1934 refurbishment, it was reported that "several globules of aluminum, fused to the sides of the pyramid after the apex had been struck by lightning, were removed for examination and for microscopic studies." About 1/40th of a gram used for spectrographic analysis of the metal was found to contain 1.00% Fe; 0.75% Si; 0.30% Mn; 0.05% Cu; 0.02% Sn; 0.01% Na, and 97.87% Al (by difference).1
The overall lightning protection system had seemed quite adequate when designed in 1880. It consists of four hollow wrought-iron columns that stand in the well of the shaft. These act as supports for the elevator machinery and serve as a guide to the car. These columns are 15 cm in exterior diameter and slightly more than 1 cm thickness. The columns rest upon cast-iron shoes that are set in the large drum pit below the monument floor. The cast-iron shoes are connected to soft copper rods that, in turn, lead to the bottom of a water well below the masonry foundation. No disruptive discharge of electricity was experienced between 1880 and 1884.
When the pyramidion was completed with the setting of the top stone in December 1884, four copper rods about 2 cm in diameter were run from each column to the top stone. There they were united with a 3.8 cm diameter copper rod that passed vertically through the top stone and screwed into the terminal aluminum apex. The entire system was completed, tested, and connected and its resistance checked on January 20, 1885.8
In June of that year, however, an incident occurred that forced another look at the protection system. In his report to the chief of engineers following the incident, Casey said:
"On the 8th of June 1885, during a thunderstorm, a disruptive discharge was seen to pass between the summit of the pyramidion and the cloud. Upon examining the structure, a crack was discovered in the north face of the pyramidion just under the top stone. A small piece was forced outward 3/4 of an inch. It was forced back into place and bolted to the solid stone from which it had been torn."8This event caused considerable concern and resulted in Casey seeking the advice of professional consultants from John Hopkins University, the U.S. Navy, and the U.S. Signal Service. Upon examining the monument and appraising the conditions, modifications were recommended and implemented. This consisted of four 1.27 cm copper rods, fastened by a copper band to the aluminum terminal and led down the corners of the pyramidion (approximately 18 m), then passed through the masonry and joined to the internal iron columns of the elevator shaft. Numerous other copper rods were added in the interconnecting network. All of the added exterior copper rods and bands were gold plated and studded every 1.5 m of their lengths with copper points 7.6 cm in length, gold plated, and tipped with platinum.8 Eight points were also added to the top edge of the copper band mechanically affixed to the aluminum pyramid. These are visible in Figure 2.
Figure 2. A depiction of 1885 modification to the lightning rod system with gold-plated copper bar girdling the aluminum pyramid near its base and s erving as an attachment point for gold-plated copper rods added to full-length corners of the pyramidion.
Figure 3. An 1885 photograph of the construction platform used to complete the pyramidion. (Source: National Archives, record group 42.)
Figure 4. The 1934 refurbishment with workmen setting larger rods into the copper bar girdle.
At this juncture, it was of no consequence whatsoever that the reason given by Casey for choosing aluminum for the apex in 1884 was "because of its whiteness and the probability that its polished surfaces would not tarnish upon exposure to air."8 Essentially this was achieved since the original 1884 inscriptions were still readable in 1934.1
The obvious question this raises is why was aluminum not used in 1885 for the four rods run down the external corners to the base of the pyramidion? Additionally, these were tied with more such rods that circled its girth in several locations. There are several answers: aluminum was not commercially available in that size of rod form at that time, while copper rods had long been a market commodity; the amount of aluminum needed was about 59.6 kg while Frishmuth's total 1885 production was only 28.3 kg spread throughout the year (aluminum could have come from France where there was plenty of it, but it would have taken months to arrive); and the girdle bar of copper fastened by set screws to the aluminum pyramid was added to facilitate the connection of the added copper rods on the edges to the central conducting conduit that included the aluminum pyramid.
Figure 5. Polished and engraved replica of the aluminum pyramid cast in the same foundry 100 years later\emdash November 12, 1984. The two views show the engraving on the four sides of the pyramid.
Figure 6.The ribbon-cutting ceremony at Tiffany's at their New York location on November 15, 1984.
Figure 7. The Frishmuth foundry building in 1983 operating under the name Eastern Nonferrous Foundry. The street signs are made of aluminum.
ABOUT THE AUTHOR
George J. Binczewski earned his B.S. in metallurgy at the University of Pennsylvania in 1953 and his MBA at Gonzaga University in 1965. He is currently a principal at SCSystems. Mr. Binczewski is also a member of TMS.
For more information, contact G.J. Binczewski at SCSystems, P.O. Box 6154, Moraga, California 94570; (510) 284-5007; fax (510) 376-5592.
The 1884 price of aluminum was approximately $1 per ounce, the same as the then prevailing market price of silver, which was considered a precious metal. The world production of new mine silver in 1884 was approximately 2,834 tonnes. Best reported estimates for world aluminum production in 1884 were 3.6 tonnes, most of it in France and England and some in Germany. Frishmuth's Philadelphia works, the only U.S. producer at that time, is given credit for production of 51 kilograms in 1884. Thus, with the thousand-fold difference in new production between the two metals we might expect that the economic laws of supply would foster a much higher price for aluminum than for silver; however, the demand factor of the same economic law (or the lack of demand) had kept the price of aluminum fairly static for the previous 20 years.
Aluminum's relatively high price of $1 per ounce ($16 per lb.) was directly related to the high cost and difficult chemical reduction process then in use to produce it. Anhydrous aluminum chloride was reduced by sodium metal at elevated temperatures by the reaction
Al2Cl6(g) + 6Na(l) = 2Al(s) + 6NaCl(s)
It was costly to produce anhydrous aluminum chloride from aluminum oxide. Also, high-cost sodium had to be produced by electrolysis of molten sodium hydroxide. Combining and containing the materials at the elevated temperatures required for the reaction was a big problem.
In 1854, H. Saint Clair Deville announced the success of the sodium reduction process for obtaining sizable quantities of aluminum in a rather straightforward technical, but difficult commercial, manner. One might expect that the many favorable attributes of the metal would provide the impetus for its accelerated use in the products of commerce, which in turn would foster a rapid increase in its production. But that did not happen, and from 1854 to 1884 the industry, principally located in France, languished while its pioneers struggled to overcome the many problems that its development presented.
The summation of the industry's overall difficulties is best described by a knowledgeable and deeply involved industry practitioner, Clemens Winckler, who was reported in the Scientific American2 in 1879 as writing, "There are several reasons why the metal is shown so little favor....First of all there is the price; then the methods of working it are not everywhere known; and further, no one knows how to cast it."
Winckler's 1879 vantage point provides the insight for the lack of a developed vitality of the industry. This was the legacy of aluminum's producers 25 years after Deville's sodium process announcement. It also highlights the achievement of William Frishmuth who, a short five years later, produced the largest casting of the metal ever made up until that time.
A more critical interpretation of Winckler's remark concerning casting would relate it to the characteristics of the metal in the liquid state during his time. First, there was the erratic chemical composition yielded by the sodium reduction process—seldom better than 97% with the primary impurities of iron and silicon well above 1% each. Also, the physical impurity content was high and of an unknown character to the early practitioners. Gas content was present but hardly recognized. Deville3 describes a purifying process involving as many as four meltings or more, cautioning his readers not to be fooled by the seemingly good brightness and color that the metal may display after the first melting and solidification process.
Fifty years later, and as late as 1930, Dix4 affirmed the poor casting qualities of pure aluminum and suggested the alloying with other elements for this reason and to improve its strength. Indeed, the bane of the well-developed casting industry during the period 1910-1930 was the high percentage of wastes encountered. This was new cast metal that had to be remelted, allowing for the fact that during this time increasingly complicated shapes were being produced.
Having completed his studies as a practical chemist, Frishmuth traveled extensively through the Caribbean, the United States, and South America. He finally settled in the United States, became a citizen, and made Philadelphia his home in 1855. His first years in his new city were devoted to chemistry and the production of rare and alkaline metals. Through 1859, his production was reported to be 64.4 kg of sodium, about 10 kg of potassium, and more than 13 kg of aluminum.
By 1860, he had become increasingly interested in politics and was a very active anti-slavery advocate. During the 1860 presidential campaign, he supported Abraham Lincoln on the emancipation issue and traveled the mid-Atlantic and New England states addressing the German-speaking communities on his behalf. This effort resulted in the close acquaintance of Lincoln, who took a liking to him and invited him to Washington, D.C., for the presidential inaugural. When the Civil War began, Frishmuth was appointed as a special secret agent in the U.S. War Department by the Secretary of War at the request of Lincoln. In 1861 he was reportedly awarded $200 in gold from Lincoln's private purse for capturing three spies.
Frishmuth was given permission by Lincoln to raise a regiment of cavalry for active army service.11 This action was sanctioned by Governor Curtin of Pennsylvania and resulted in Frishmuth organizing the 12th Pennsylvania Volunteer Regiment of Cavalry, which became known as Frishmuth's Cavalry.12 It was accepted by Lincoln for federal service with Frishmuth commissioned as colonel. But shortly afterwards, disagreements and troubles with minor officers resulted in Frishmuth resigning his commission.12 He was immediately reappointed as a special agent by Lincoln.
After the Civil War, Frishmuth studied law and was appointed commissioner of deeds by the Governor of New Jersey. He was commissioned as a notary public by Governor Geary of Pennsylvania, who also gave him the commission of colonel of the First Regiment Cavalry in the National Guard of Pennsylvania.
In the 1870s he once again became involved in the chemistry profession, became active in electroplating of metals, and was engaged in improving methods to produce aluminum more cheaply. He was later involved in methods to improve electrolighting, the design of a new battery, and a portable electric lamp. He received a total of 12 U.S. patents in these various endeavors and also pursued and received patents in other countries. A chronological listing of U.S. patents granted to Frishmuth presents evidence of his technical expertise and versatility (Table 1).
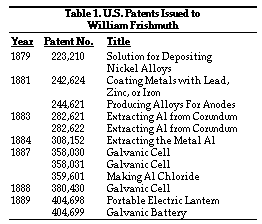
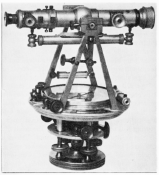
Figure A. An elaborate aluminum surveyor\rquote s transit made in Troy, New York, with the metal source attributed to Wm. Frishmuth Manufacture. It was exhibited at the 1876 Centennial Exposition.
Frishmuth, like most inventors, was quite secretive about disclosing specific details of his process. He was always seeking process backers and many of his patent assignments reflect this. Perhaps the most revealing status of Frishmuth's achievement is contained in a 1,500 word article that appeared in the November 25, 1884, edition of the New York Times. The article's final paragraph presents a most favorable and optimistic assessment. It reads,
"The control of the patents for the new process has been secured by foreign capitalists, who will thus have the whole world for their market. Their intentions are understood to be similar to the policy pursued by the Rothchilds, who control the Quicksilver mines of the world, viz to feed the world with a product at a reasonable price, and regulate the supply by the demand. The English capitalists controlling the patents some time ago sent over Major Ricardo Seaver, F.R.S.F., late Government Inspector General of Mines, of London, who made a thorough examination of the process. He returned home a few weeks ago to make his report. He is said to have been thoroughly satisfied with the practicability of the process and its successful application to the needs of the world. As showing what has been done at the Philadelphia works, a handsome collection of the metal in bulk and articles manufactured from it and its alloys has been prepared and sent to New Orleans for exhibition at the coming exposition. At present anyone in New York or vicinity wishing to see a specimen of the product can do so by visiting Tiffany's, where the apex for the Washington Monument at the National Capitol, manufactured by order of the Government, is now on exhibition."Frishmuth has not been accorded the honor and recognition for his manifold achievements and contributions. His technological accomplishments are exemplified by his 12 patents in the fields of electroplating, aluminum production and casting, galvanic batteries, and portable electric lights. His strong socio-political convictions and service to President Abraham Lincoln are a testament to his character. His business acumen and public relation activities indicate a perceptive person who knew how to deal with people.
By the early 1890s, the chemical reduction method for making aluminum had succumbed to the new low-cost electrolytic process of C.M. Hall. Frishmuth became the victim of a combination of business, legal, and personal difficulties. On the morning of August 1, 1893, William Frishmuth was found in his Philadelphia home at Frankford Avenue and Tioga Street dead from a self-inflicted gunshot wound in the head.
It is a notable aside that all of the correspondence on this subject in the National Archives files is in handwritten form, even received Western Union telegrams. While various models of typewriters had been in commercial use by 1884, they had not yet been standardized and were too expensive for government agencies or small foundries such as Frishmuth's.
The ceremony for the placement of the aluminum point appeared as a large 17 cm by 25 cm sketch in the center of the front page of the Washington Post 16 hours after the fact in its December 7, 1884, edition. This picture has been reproduced thousands of times and is today prominently displayed whenever the monument is described. It appeared as a finely detailed, five-color lithograph in the December 20, 1884, issue of Harper's Magazine. [The sketch is reproduced on the cover of this issue.—ed.] While the art of photography was well established by 1884, the gale force winds and driving rain at the 169 meter platform level precluded the taking of an actual photo. An artist's sketch of the event had to suffice.
Frishmuth's public relations savvy and skills are exemplified by his ability to get the attention of the famous Tiffany's jewelry store in New York. In late November 1884, Tiffany's displayed the polished aluminum pyramid for the benefit of "thousands of New Yorkers who delighted in being able to later say `I stepped over the top of the Washington Monument."13
Frishmuth created much consternation for Casey by delaying the delivery of the pyramid to Washington, D.C. while he publicized and displayed it in different places. After Casey literally threatened him, Frishmuth sent the pyramid with a letter request to Casey to exhibit the point in the House and the Senate.9
Frishmuth's self satisfaction and pride in his accomplishment is
exemplified by his request to Casey to have the pyramid wiped with a chamois to
remove fingerprints after it had been set in place atop the
monument.9
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|