 |
48, (4) (1996), pp. 50-54. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
 |
48, (4) (1996), pp. 50-54. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
Edited by Garry W. Warren of the University of Alabama at Tuscaloosa, EPD Congress 1996 collects papers presented during several of the symposia sponsored in large part by the TMS Extraction & Processing Division (EPD) during February at the 1996 TMS Annual Meeting in Anaheim, California. Each year, the EPD volume features an array of single- and multi-session symposia on topics related to the processing of metals from a variety of perspectives, including the processing of ores and concentrates, primary production, and the recovery from scrap and by-product materials. The 1996 EPD Congress proceedings volume contains 78 papers from nine symposia—General Pyrometallurgy; Hydrometallurgy; Recent Developments; Process Fundamentals; Process Mineralogy; General Recycling; Treatment of Bulk Concentrates and Residues in Lead-Zinc-Tin Production; Materials Processing Fundamentals; Concurrent Engineering; and the International Symposium on the Technical, Economic, Commercial, and Environmental Consequences of Sulfur Recovery in Copper Production Systems.
As has become an annual tradition for JOM, the following is designed to graphically provide a sampling of the types of technologies that were presented during the congress. All of the papers highlighted here (titles appear in parentheses) appear in the EPD Congress 1996.
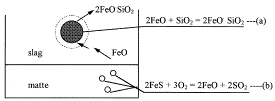 The converting of copper mattes requires the use of silica flux to generate the
ferrous silicate slag in which the iron in the matte is removed. Poor fluxing
procedure can result in the generation of solid magnetite during converting
operations; the increasing popularity of high-matte grades has made avoiding this
problem more challenging than before. Jian, An, and Hendrix of the University of
Nevada at Reno ("Melting Behavior of Silica Flux in the Copper Converter")
examined the rate at which silica particles melt in contact with molten matte.
Lower particle size was the most important factor in determining melting rates;
increased matte grade also had a significant impact. However, blast flow rate and
oxygen content in the blast made little difference.
The converting of copper mattes requires the use of silica flux to generate the
ferrous silicate slag in which the iron in the matte is removed. Poor fluxing
procedure can result in the generation of solid magnetite during converting
operations; the increasing popularity of high-matte grades has made avoiding this
problem more challenging than before. Jian, An, and Hendrix of the University of
Nevada at Reno ("Melting Behavior of Silica Flux in the Copper Converter")
examined the rate at which silica particles melt in contact with molten matte.
Lower particle size was the most important factor in determining melting rates;
increased matte grade also had a significant impact. However, blast flow rate and
oxygen content in the blast made little difference.
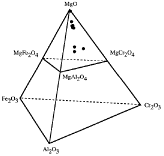 Most copper and nickel smelters are heavy users of mag-chrome refractories of
varying types. Fukuyama, Donald, and Toguri of the University of Toronto
("Wetting Behavior and Interfacial Reaction Between Fayalite Slag and MgO")
presented two papers focusing on possible chrome-free alternatives. The diagram
illustrates the compositions of various refractories in the
MgO-Cr2O3-Fe2O3-Al2O3
system tested in contact with fayalite slag to determine how refractory
composition affected the wetting angle and interfacial reaction with the
fayalite slag. MgO showed low wettability and good chemical resistance to the
slag; the formation of an immediate magnesiowüstite layer at the
refractory surface helped retard reaction rates, thus reducing corrosion.
Most copper and nickel smelters are heavy users of mag-chrome refractories of
varying types. Fukuyama, Donald, and Toguri of the University of Toronto
("Wetting Behavior and Interfacial Reaction Between Fayalite Slag and MgO")
presented two papers focusing on possible chrome-free alternatives. The diagram
illustrates the compositions of various refractories in the
MgO-Cr2O3-Fe2O3-Al2O3
system tested in contact with fayalite slag to determine how refractory
composition affected the wetting angle and interfacial reaction with the
fayalite slag. MgO showed low wettability and good chemical resistance to the
slag; the formation of an immediate magnesiowüstite layer at the
refractory surface helped retard reaction rates, thus reducing corrosion.
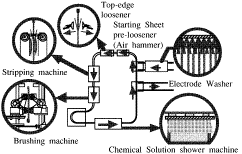 As the value of the yen increases, the need to reduce labor costs in
Japanese manufacturing facilities is increasingly important. Oda, Kusano, and
Noda of Hibi Kyoudou Smelting ("Recent Operation in Tankhouse of Tamano
Smelter") discussed the modernization of the tankhouse at their company's
copper production facility. One of the labor-saving devices adopted during the
project was the automated stripping process for starter sheets shown here. This
allowed the number of operators needed to be reduced from three to one. "Fuzzy
guesswork" control is used to help control the swinging of mother blanks as
they are transferred between locations in the process.
As the value of the yen increases, the need to reduce labor costs in
Japanese manufacturing facilities is increasingly important. Oda, Kusano, and
Noda of Hibi Kyoudou Smelting ("Recent Operation in Tankhouse of Tamano
Smelter") discussed the modernization of the tankhouse at their company's
copper production facility. One of the labor-saving devices adopted during the
project was the automated stripping process for starter sheets shown here. This
allowed the number of operators needed to be reduced from three to one. "Fuzzy
guesswork" control is used to help control the swinging of mother blanks as
they are transferred between locations in the process.
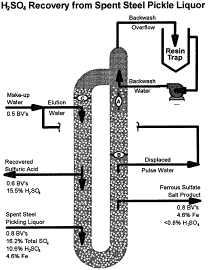 The recovery of metallic impurities from waste solutions is an
increasingly common practice, but the recovery of acid content from these
streams is a new development. R.S. Dennis of Tetra Technologies ("Acid Recovery
from Spent Acids and Electrolytes via Continuous Ion Exchange") discussed new
technology for acid recovery from waste streams using strong-base anion
exchange resins. The figure illustrates the recovery of ferrous sulfate and
sulfuric acid from spent pickle liquor generated by steel plants; the new
technology recovers more than 93 percent of the sulfuric acid from the spent
liquor at close to its original strength. The process has been applied to a
variety of other waste streams as well.
The recovery of metallic impurities from waste solutions is an
increasingly common practice, but the recovery of acid content from these
streams is a new development. R.S. Dennis of Tetra Technologies ("Acid Recovery
from Spent Acids and Electrolytes via Continuous Ion Exchange") discussed new
technology for acid recovery from waste streams using strong-base anion
exchange resins. The figure illustrates the recovery of ferrous sulfate and
sulfuric acid from spent pickle liquor generated by steel plants; the new
technology recovers more than 93 percent of the sulfuric acid from the spent
liquor at close to its original strength. The process has been applied to a
variety of other waste streams as well.
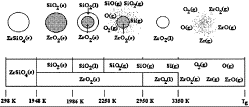 The zirconia content in zircon (ZrSiO4) is increasingly valuable for
several high-technology applications. However, separating the zirconia from the silica
in zircon remains a challenge. Manrique, Gomez, and Taylor ("Thermal
Decomposition of Zircon in a Non-Transferred Arc Thermal Plasma Flow Reactor")
discussed the results of a joint project between the Venezuelan Simon Bolivar
University and the University of Idaho investigating the fundamentals behind
the plasma-based melting and dissociation of zircon. The high temperatures
generated by the plasma unit progressively decompose zircon particles as shown;
when the vapors reoxidize as they cool elsewhere in the reactor, a
submicrometer-size mixture of zirconia, silica, and zircon particles results.
The higher vapor pressures of SiO help encourage the separation of the silica
from the zirconia, providing the basis for a potential commercial process.
The zirconia content in zircon (ZrSiO4) is increasingly valuable for
several high-technology applications. However, separating the zirconia from the silica
in zircon remains a challenge. Manrique, Gomez, and Taylor ("Thermal
Decomposition of Zircon in a Non-Transferred Arc Thermal Plasma Flow Reactor")
discussed the results of a joint project between the Venezuelan Simon Bolivar
University and the University of Idaho investigating the fundamentals behind
the plasma-based melting and dissociation of zircon. The high temperatures
generated by the plasma unit progressively decompose zircon particles as shown;
when the vapors reoxidize as they cool elsewhere in the reactor, a
submicrometer-size mixture of zirconia, silica, and zircon particles results.
The higher vapor pressures of SiO help encourage the separation of the silica
from the zirconia, providing the basis for a potential commercial process.
 The use of sodium carbonate for removing antimony and arsenic from molten
copper is an increasingly popular fire-refining procedure. Zhao and Themelis
from Columbia University ("Kinetics of As and Sb Removal from Molten Copper by
Na2CO3 Fluxing") described the results of a laboratory
investigation on how the rate at which this reaction occurs could be improved. Arsenic and antimony
removal is controlled by mass transfer of these impurities through the metal
phase. A model was developed expressing the removal rate as a function of
several controllable variables, including bath agitation,
Na2CO3
feed rate, and oxygen content in the molten copper. The location of soda-ash addition was also
important, with injection producing quicker results than surface addition.
The use of sodium carbonate for removing antimony and arsenic from molten
copper is an increasingly popular fire-refining procedure. Zhao and Themelis
from Columbia University ("Kinetics of As and Sb Removal from Molten Copper by
Na2CO3 Fluxing") described the results of a laboratory
investigation on how the rate at which this reaction occurs could be improved. Arsenic and antimony
removal is controlled by mass transfer of these impurities through the metal
phase. A model was developed expressing the removal rate as a function of
several controllable variables, including bath agitation,
Na2CO3
feed rate, and oxygen content in the molten copper. The location of soda-ash addition was also
important, with injection producing quicker results than surface addition.
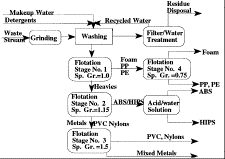 Someday your dishwasher will go the way of all dishwashers. When it does,
recovering the plastics used to put it together will be just as important as
recovering the metal parts. Karvelas et al. at the Energy Systems Division of
Argonne National Laboratory ("Recovery and Separation of High Value-Plastics
from Discarded Household Appliances") have been working on the recycling of
household appliances and have developed a process for separating one type of
plastic from another. A variety of minerals processing techniques are involved,
relying largely on the difference in the specific gravities of the different
plastics. Research is currently underway to find a means of separating the
high-impact polystyrene in the waste stream from the
acroylonitrile-butadiene-styrene, which has roughly the same specific gravity
and cannot be separated by ordinary flotation techniques.
Someday your dishwasher will go the way of all dishwashers. When it does,
recovering the plastics used to put it together will be just as important as
recovering the metal parts. Karvelas et al. at the Energy Systems Division of
Argonne National Laboratory ("Recovery and Separation of High Value-Plastics
from Discarded Household Appliances") have been working on the recycling of
household appliances and have developed a process for separating one type of
plastic from another. A variety of minerals processing techniques are involved,
relying largely on the difference in the specific gravities of the different
plastics. Research is currently underway to find a means of separating the
high-impact polystyrene in the waste stream from the
acroylonitrile-butadiene-styrene, which has roughly the same specific gravity
and cannot be separated by ordinary flotation techniques.
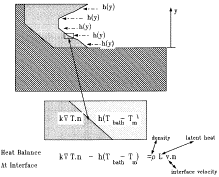 A common practice in pyrometallurgical operations is to deliberately freeze a
portion of a molten slag or bath against the furnace wall. This protects the
wall from corrosion by its molten contents and lengthens the furnace's life
span. Voller at the University of Minnesota ("Modeling of Ledges and Skulls")
is using finite-element analysis to model the formation of such ledges based on
the heat balance at the solid-liquid interface as shown above. Such modeling
will allow the prediction of changes in the shape and thickness of ledges as
furnace conditions change, along with the calculation of heat losses from the
furnace as the thickness and profile of the ledge changes. The work is in its
early stages, but shows considerable promise as a useful tool for the
management of pyrometallurgical furnace operations.
A common practice in pyrometallurgical operations is to deliberately freeze a
portion of a molten slag or bath against the furnace wall. This protects the
wall from corrosion by its molten contents and lengthens the furnace's life
span. Voller at the University of Minnesota ("Modeling of Ledges and Skulls")
is using finite-element analysis to model the formation of such ledges based on
the heat balance at the solid-liquid interface as shown above. Such modeling
will allow the prediction of changes in the shape and thickness of ledges as
furnace conditions change, along with the calculation of heat losses from the
furnace as the thickness and profile of the ledge changes. The work is in its
early stages, but shows considerable promise as a useful tool for the
management of pyrometallurgical furnace operations.
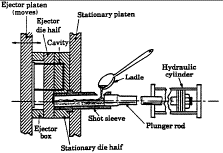 The illustration of a horizontal die-casting process shows the position of a
shot sleeve—a vital part in the proper injection of the molten metal into the
die chamber. Shot sleeves deteriorate quickly and fail in service frequently,
so the need for an improved design process for these parts is apparent. Park
and Brevick at Ohio State University ("Concurrent Engineering of Die Casting
Process Parameters and Tooling") have been working on such a design process,
focusing on the use of martensitic H13 steel. The impact of heat-treatment
temperature on the mechanical properties and critical crack size of the steel
is important in being able to predict the life span of shot sleeves as a
function of their thickness and the temperatures to which they are cyclically
subjected.
The illustration of a horizontal die-casting process shows the position of a
shot sleeve—a vital part in the proper injection of the molten metal into the
die chamber. Shot sleeves deteriorate quickly and fail in service frequently,
so the need for an improved design process for these parts is apparent. Park
and Brevick at Ohio State University ("Concurrent Engineering of Die Casting
Process Parameters and Tooling") have been working on such a design process,
focusing on the use of martensitic H13 steel. The impact of heat-treatment
temperature on the mechanical properties and critical crack size of the steel
is important in being able to predict the life span of shot sleeves as a
function of their thickness and the temperatures to which they are cyclically
subjected.
 -NiAl and CdTe Crystals" by H. Ouyang and W. Shyy
-NiAl and CdTe Crystals" by H. Ouyang and W. Shyy
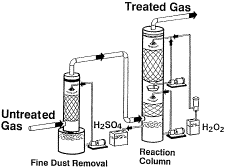 The desulfurization of weak SO2 gas streams has always
been a problem. Solid absorbents have usually been employed for SO2
removal from these streams, but there is room for improvement. Steiner at Degussa
Corporation ("Off-Gas Desulfurization with Hydrogen Peroxide") discussed the
practice of treating weak off-gas streams by reaction with hydrogen peroxide.
The reaction generates a sulfuric acid solution, removing nearly all of the
SO2 from the off-gas. Scrubbing the off-gas with water prior to
H2O2
treatment removes most of the metal content, resulting in an acid product suitable for
use as battery acid. The process has been in commercial use for more than a
decade in a variety of production environments.
The desulfurization of weak SO2 gas streams has always
been a problem. Solid absorbents have usually been employed for SO2
removal from these streams, but there is room for improvement. Steiner at Degussa
Corporation ("Off-Gas Desulfurization with Hydrogen Peroxide") discussed the
practice of treating weak off-gas streams by reaction with hydrogen peroxide.
The reaction generates a sulfuric acid solution, removing nearly all of the
SO2 from the off-gas. Scrubbing the off-gas with water prior to
H2O2
treatment removes most of the metal content, resulting in an acid product suitable for
use as battery acid. The process has been in commercial use for more than a
decade in a variety of production environments.
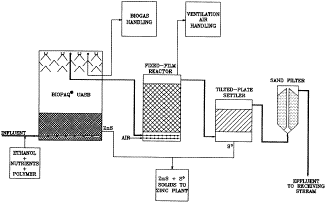 Another problem area in sulfur removal has been acid-mine waste. De Vegt and
Buisman ("Sulfur Compounds and Heavy Metal Removal Using Bioprocess
Technology") introduced a new process for sulfur recovery from mine waters that
relies on biological processing to precipitate metal sulfides and elemental
sulfur. Their paper discussed the results obtained from a pilot-plant
experiment at the Budelco zinc refinery in Holland. The process was highly
effective at removing trace-metal content from groundwater at the site in
addition to eliminating more than 80 percent of the sulfate content. The new
process has potential value in treating a variety of liquid and gaseous
sulfur-bearing waste streams.
Another problem area in sulfur removal has been acid-mine waste. De Vegt and
Buisman ("Sulfur Compounds and Heavy Metal Removal Using Bioprocess
Technology") introduced a new process for sulfur recovery from mine waters that
relies on biological processing to precipitate metal sulfides and elemental
sulfur. Their paper discussed the results obtained from a pilot-plant
experiment at the Budelco zinc refinery in Holland. The process was highly
effective at removing trace-metal content from groundwater at the site in
addition to eliminating more than 80 percent of the sulfate content. The new
process has potential value in treating a variety of liquid and gaseous
sulfur-bearing waste streams.
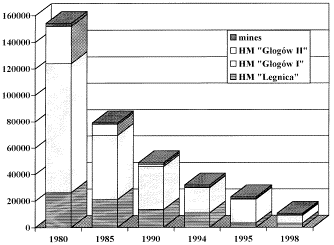 The figure illustrates the dramatic progress made over the past 15 years in
reducing SO2 emissions from the two Polish copper smelters in the
Legnica-Glogów region, as discussed by Byrdziak et al. ("Sulfur Dioxide
Emission Reduction by the Polish Copper Industry"). The most recent
improvements include installation of a new desulfurization facility at Legnica,
which began operation in 1993. The new facility features a single-stage,
contact-acid plant followed by subsequent treatment using the Solinox process.
The SO2 absorbed by the organic solvent used in this second step is
easily regenerated, although additional scrubbing was eventually required for chloride
removal. A new desulfurization facility at Glogów is also under
consideration.
The figure illustrates the dramatic progress made over the past 15 years in
reducing SO2 emissions from the two Polish copper smelters in the
Legnica-Glogów region, as discussed by Byrdziak et al. ("Sulfur Dioxide
Emission Reduction by the Polish Copper Industry"). The most recent
improvements include installation of a new desulfurization facility at Legnica,
which began operation in 1993. The new facility features a single-stage,
contact-acid plant followed by subsequent treatment using the Solinox process.
The SO2 absorbed by the organic solvent used in this second step is
easily regenerated, although additional scrubbing was eventually required for chloride
removal. A new desulfurization facility at Glogów is also under
consideration.
For more information on acquiring EPD Congress 1996, contact TMS at 184 Thorn Hill Road, Warrendale, Pennsylvania 15086; (724) 776-9000, ext. 270; fax (724) 776-3770; e-mail csc@tms.org; you can also visit the following World Wide Web address: http://www.tms.org/pubs/Books/EachBook/3104.html.
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|