 |
49 (7) (1997), pp. 14-21, 83. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
 |
49 (7) (1997), pp. 14-21, 83. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
| CONTENTS |
|---|
|
|
Hot demonstrations with actual radioactive waste inevitably reveal a number of new process and equipment issues that have not come to light during cold development in the laboratory with surrogate materials. Sound engineering solutions to these problems are required before a new process can be made viable for application at an industrial scale. The demonstrations at ANL-W are designed to provide this necessary link in the development of waste treatment processes before substantial funds are risked on their deployment. Bringing to bear most of the baggage of nuclear regulation, hot demonstrations are not inexpensive. But by taking advantage of existing nuclear facilities, engineering expertise in remotely operated and maintained equipment, and an extensive complement of analytical and on-line measurement capabilities, the time and cost required for the demonstration phase of the development cycle can be minimized.
By far the most extensive waste-processing demonstration is a key part of the Electrometallurgical Treatment Research and Demonstration Project sponsored by the DOE Office of Nuclear Energy Science and Technology.4 Within the DOE complex, there are some 53 categories of spent nuclear fuel for which disposition options are being evaluated.5 Many types of fuel may eventually be directly disposed in a geological repository as proposed for commercial spent nuclear fuel, but some will require treatment prior to disposal.
Fuel containing sodium as a thermal bond between the fuel matrix and cladding is the most certain to require treatment. Elemental sodium is regulated under the Resource Conservation and Recovery Act (RCRA) as a hazardous material because of its chemical reactivity. Much of the existing sodium-bonded spent nuclear fuel was used in the Experimental Breeder Reactor II (EBR-II) at ANL-W. An electrometallurgical technique is being developed by ANL for processing this fuel and producing stable waste forms for disposal. The development of the high-level waste forms actually started in 1985 as part of the Integral Fast Reactor Program,6 which was canceled in 1994. Waste-form development is expected to continue through 1999, with equipment demonstrations and hot sample testing into the next century.
One of the largest waste management problems in the DOE complex is the disposition of the tens of thousands of cubic meters of plutonium-contaminated waste that was generated during operation of the nuclear weapons production facilities. Much of this waste is designated as transuranic (TRU) waste for its content of transuranium elements. The remaining waste in this class, with concentrations of transuranics between 10 nCi/g and 100 nCi/g, is designated as alpha low-level waste. TRU waste is eligible for deep geologic disposal when the Waste Isolation Pilot Plant opens in New Mexico, but the alpha low-level mixed waste is an orphan waste. More often than not, the waste contains hazardous materials, which further complicates its regulation and disposal. Thermal processing to eliminate the hazardous component and reduce the volume has been suggested as an appropriate treatment for plutonium-contaminated wastes prior to disposal. This approach has been selected for approximately 60,000 m3 of TRU and alpha low-level waste stored in Idaho.7
Engineers at ANL-W are demonstrating a high-temperature thermal process thought to be suitable for the treatment of a wide variety of DOE mixed waste, although it is not the technology selected for treatment of the Idaho TRU waste. The plasma-hearth process (PHP) produces a reduced-volume, highly stable waste form for disposal. Under the sponsorship of the DOE's Mixed Waste Focus Area, the PHP benchscale demonstration being conducted at the ANL-W site is one of several key projects comprising the disciplined approach taken to development of this technology. The benchscale project is a joint undertaking of ANL and Science Applications International Corporation, which has tested a nonradioactive benchscale unit.8 A pilot-scale, nonradioactive unit is being tested at Retech9 in Ukiah, California.
The third waste processing demonstration involves the application of a type of material called chemically bonded phosphate ceramics (CBPCs) for stabilizing radioactive and RCRA-regulated wastes. CBPCs could potentially be used for many of the same waste streams as the PHP, but may also complement technologies such as the PHP by stabilizing the ash and volatile residues from thermal processes. In principle, the CBPC approach is simpler and less expensive than the PHP, but does not achieve the volume reduction attributed to thermal-treatment processes. CBPC stabilization of two different types of waste streams is being demonstrated. One demonstration, involving crushed glass from mercury vapor lamps used in hot cells, is being sponsored by the DOE's Mixed Waste Focus Area. The second demonstration, involving TRU-bearing incinerator ash, is being sponsored by the DOE's Plutonium Focus Area. In comparison to the other high-level waste and PHP demonstrations, the CBPC demonstrations are simple, quick, and inexpensive. The process equipment involves nothing more sophisticated than a mixer used in restaurant kitchens.
None of the three ANL-W demonstrations quite meet the common industrial notion of a process demonstration, although the PHP benchscale test comes close. In each case, too little is known about the final waste form and the performance of the process when confronted with real radioactive waste streams and all their inherent complexity. Each program involves substantial R&D components, as there is no significant radioactive experience with any of the processes being demonstrated. The goal of each program is to conduct experiments under prototypic radioactive conditions, develop improvements in process parameters and equipment, and ultimately demonstrate a unit process operation that is readily adaptable or scalable for large-scale deployment.
The central component of electrometallurgical-treatment technology10,11 is an electrorefiner containing molten chloride salts at 500°C. Chopped spent-fuel rods are fed into the vessel in steel baskets, and an electric potential is applied between the anode baskets and a steel mandrel cathode. The fuel matrix material is dissolved out of the cladding, and relatively pure uranium metal is deposited on the cathode. The more chemically active fission products (alkali, alkaline earths, and rare earths) are oxidized as chlorides in the salt, while the more noble fission products remain with the cladding hulls. The TRUs are primarily in the salt phase.
From this process, two high-level waste (HLW) forms are produced. The cladding hulls and noble fission products are consolidated into a durable stainless steel, zirconium-based ingot called the metal waste form. The active fission products are removed from the salt by ion exchange in zeolite. The zeolite is combined with glass frit and processed into a solid monolith using a hot isostatic press (HIP). The zeolite-based waste is called the ceramic waste form, also known as glass-bonded zeolite or the mineral waste form.
The fuel-treatment technology and its associated waste forms are being developed in parallel. The electrometallurgical technique is very different from typical aqueous reprocessing. It is a compact process, requires minimal chemical additives, and produces the same types of compact HLW forms for any type of fuel processed. The waste forms have been developed to be directly compatible with the chloride and metallic waste streams.
The treatment technology and waste forms have followed the same development approach. First, small laboratory-scale tests are performed using nonirradiated materials. Next, engineering-scale tests are performed with nonirradiated materials. Finally, the process is demonstrated using irradiated materials in engineering-scale equipment. The laboratory-scale development of the waste forms is being done by the Chemical Technology Division at the ANL site in Illinois, while the engineering-scale tests and the demonstration with actual HLW are being performed primarily at ANL-West. Of the EBR-II spent fuel assemblies, 125 will be treated in the hot cells of the Fuel Conditioning Facility at ANL-W. Operations with irradiated fuel began in June 1996. Engineering-scale work with nonirradiated materials began in early 1996.
Zeolites are crystalline aluminosilicates of the group I (alkali) and group II (alkaline earth) elements. Their framework is a network of AlO4-4 and SiO4-4 tetrahedra linked by the sharing of oxygen atoms. The specific zeolite being tested in the ceramic waste form is zeolite A, Na12[(AlO2)12(SiO 2)12]. The networks of tetrahedra in the zeolite form cages in which molecules are occluded. The sodium ions in this structure are subject to ion exchange. Both of these properties are used in producing the ceramic waste form. Natural sodalite, into which zeolite A can be converted, retains chloride ions very readily. Na8[(AlO2)6(SiO2)6]Cl2 is the chemical form of sodalite. A zeolite-based waste form is compatible with fission-product chlorides and was a natural selection for stabilizing wastes from a chloride salt electrolyte.
After the selection of a zeolite-based waste form, the laboratory-scale work focused on optimization of the waste form. Studies made to determine the appropriate zeolite led to the selection of zeolite A. Early in the development process, attention turned to a composite waste form. Because the ceramic waste would be a glass-bonded zeolite waste form, studies were made to determine an appropriate glass composition. Preparation and processing studies were also performed. Glass-loading fractions were examined. Methods for preparing salt-loaded zeolite were tested, and numerous HIP processing cycles were tested. Nonradioactive fission-product elements were used in these tests to simulate waste loading. The products from these tests were subjected to relative performance tests so that screenings could be performed to evaluate processing parameters.
From the laboratory-scale work, the process steps were developed for production of the ceramic waste form. First, the zeolite procured from vendors, which often contains more than 20 wt.% water, must be dried. The zeolite material is then contacted with the molten salts at 500°C to bind fission products into the zeolite structure. This operation can be performed by either batch contacting the salt with dried zeolite powder or using columns containing zeolite pellets or spheres. The salt-zeolite mixture, which contains essentially no free chloride (all the salt material is bound in the zeolite), is sized and mixed with glass frit. This mixture is then loaded into stainless-steel HIP cans. The cans are evacuated, sealed, and processed in the HIP. Maximum temperatures for HIP cycles range from 600°C to 750°C. Maximum pressure is typically 170 MPa. Argon is used as the pressurizing gas.
The development of the metal waste form on the laboratory scale followed a similar path. Melting these materials into a stainless steel, zirconium-based alloy was a natural choice. Many nuclear fuels are clad with stainless steel or zirconium, and their alloys have a lower melting temperature than either cladding material. Further, the fuel from the EBR-II reactor core contains a uranium-zirconium alloy. Separation of uranium from zirconium has been demonstrated.12 The zirconium is left with the stainless-steel cladding hulls that are easily fed into the metal waste form.
 |
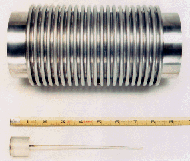
| Figure 1. The hot isostatic press (HIP) used in ceramic waste form production. |
For the ceramic waste form, laboratory-scale HIP cans were primarily 2.5 cm in diameter by either 2.5 cm or 7.6 cm in height. The sample volumes for these cans are 13 cm3 and 39 cm3, respectively. The HIP used for the engineering-scale tests is the same piece of equipment that will be placed in a remote hot-cell environment for demonstration tests with irradiated materials (Figure 1). The cans are 11 cm in diameter and 23 cm tall with a volume of 2,300 cm3, 177 times larger than the smallest laboratory-scale can. Figure 2 depicts the two extremes in can size. Because of the large scale-up and the fact that the next stage of operations requires moving from hands-on testing to remote operations, the cold engineering-scale tests are crucial to solving scale-up problems.
 |
| Figure 2. Laboratory-scale and engineering-scale HIP cans. |
The preparation equipment for the engineering-scale tests was scaled in a two-step process. The zeolite drying operation and batch salt contacting with zeolite were initially scaled by a factor of seven from the laboratory scale. (The goal for the demonstration with irradiated materials was to increase the scale by a factor of almost 50.) Batch-contacting salt with zeolite is a unique process in that no off-the-shelf equipment exists for this scale of operation. The process starts by mixing two solid powders at ambient temperature. As the powders are intimately mixed and heated to 500-550°C, the salt phase becomes molten and is occluded in the zeolite phase. If mixed properly, the result is a single free-flowing powder with no free salt.
The intermediate-scale mixer served to bridge the gap between laboratory and engineering scales, because a completely different type of system is required at the larger scale. The laboratory-scale system employs a mixer vessel rotating within a muffle furnace. Space and heating requirements preclude the use of a muffle furnace for a system 50 times larger. The intermediate-scale mixer was instrumental in choosing the final design for the engineering-scale mixer that will be installed in the hot cells of the Hot Fuel Examination Facility at ANL-W for demonstration with irradiated materials.
Another major scale-up issue associated with the production of the ceramic waste form is the method by which HIP cans are filled. In the laboratory-scale test, the zeolite and glass frit are loaded by hand into the can through the entire can opening. A layer of powder is placed in the can and then packed using a uniaxial press. This process is repeated until the can is loaded. The lid is then welded in place. For the demonstration operation in hot cells, the flow of powders from any filling containers to HIP cans must be performed in a closed system to minimize the risk of contamination from the highly radioactive powders. Additionally, for ease of handling in a remote environment, welding the lids on the HIP cans before they are transferred into the hot cell is desirable. To meet both of these goals, the HIP cans will be filled through a 2.5 cm fill tube in the lid of the can. After evacuation of the HIP can, only the fill tube will need to be welded. This fill method eliminates the use of a uniaxial press to increase the loading density of the powders. Vibratory compaction has been tested on the engineering-scale system and will be implemented for the demonstration with irradiated materials.
As shown in Figure 2, the HIP can for the engineering-scale tests is bellowed instead of straight-walled like the laboratory-scale can. This change was made due to concerns about compression during the HIP cycle. Figure 3 is a picture of a compressed engineering-scale HIP can. The compressed can is less than 50 percent of the original volume. Bellows are used to focus the can deformation along the longitudinal axis. The use of a straight-walled can, in which most of the compression is radial, on this larger scale causes concerns about failure of the wall or welds near the lid and bottom welds of the can.
Refinement of the HIP operating cycle also requires scale-up tests. The quality of the waste form is related to the temperature experienced by the ceramic during the HIP cycle. For a given HIP cycle, the maximum centerline temperature of a 2.5 cm diameter can will not necessarily be the same as for a can with a 11.3 cm diameter. Modifications to the laboratory-scale HIP cycle, such as increasing hold times, are being tested to obtain similar temperature profiles.
Tests with specific radionuclides are being performed on both the metal and ceramic waste forms. One such test on the metal waste form is looking at the effect of technetium, one of the key radionuclides affecting the long-term performance of a geologic repository. In commercial spent nuclear fuel and borosilicate glass HLW, technetium is present as an oxide, which is soluble and mobile in ground water. In the metal waste from electrometallurgical treatment, technetium is present in an insoluble metallic form that is expected to be more stable from a repository perspective. Metal waste samples doped with a range of technetium concentrations are being prepared for study.
 |
| Figure 3. A compressed HIP can. |
As part of the demonstration of the electrometallurgical process to treat fuel, metal-waste-form samples will be produced from cladding hulls containing fission products, and ceramic-waste-form samples will be produced from electrorefiner salts containing actual fission products. The electrorefiner operates in a batch mode so cladding hulls are removed periodically from the vessel. Because the metal waste stream is available throughout the fuel treatment demonstration for sample production, work on the preparation of irradiated metal-waste-form samples has begun.
Based on the preparation of engineering-scale metal-waste-form samples with surrogate materials, the general casting recipe for irradiated materials is known, but variations in the processing parameters might be required for real waste streams. In preparing surrogate material, it is not always possible to predict what impurities might become a part of the feed material and what effect they will have on the casting process or performance of the waste form. When the cladding hulls are removed from the electrorefiner, they are coated with salt. A distillation operation is performed to remove the salt for recycle back to the electrorefiner. The efficiency of the distillation operation on real cladding hulls is not easily simulated; tests with materials that have actually been treated are crucial, because chlorine would accelerate corrosion of the metal alloy.
Electrorefiner tests with materials designed to simulate spent fuel were performed as part of the development of the electrometallurgical technique, but questions still remain as to how irradiated fuel would behave. The dissolution performance of the electrorefiner has a direct bearing on the development of the metal waste form since the materials that remain in the cladding hulls become a part of the metal waste. Areas being studied include the efficiency of uranium dissolution and the retention of noble-metal fission products and fuel-matrix zirconium under different operating parameters. This is helping to provide data for scoping the concentrations of elements in the waste stream.
The waste-stream feed for the ceramic waste form is the electrorefiner salt. During the processing of irradiated fuel, the fission products and TRUs build up in the salt as chlorides. The concentration of elements in the ceramic waste is directly related to their concentration in the salt. To provide data for waste-form performance at higher radionuclide concentrations, the bulk of the ceramic-waste-form samples will be produced toward the end of the demonstration after most of the fuel has been treated.
The information to be gained from irradiated ceramic-waste-form samples is similar to that gained on the metal waste form. How the electrorefiner operates with irradiated materials also directly affects the ceramic waste form. Prior to treatment of the electrorefiner salt to stabilize the radionuclides in zeolite, uranium present in the system as UCl3 must be removed. The degree to which uranium is separated from the fission products and TRU elements as the uranium concentration becomes lower may affect the performance of the ceramic waste. Impurities that form in the salt phase and can only be determined from actual operations will also affect the ceramic waste form.
The irradiated ceramic- and metal-waste-form samples will be extensively examined to determine their physical characteristics, including density, porosity, and mechanical properties; their microstructure will be determined from scanning electron microscopy and transmission electron microscopy. Their performance as a waste form will be determined by a number of leach tests. These data will include the effects of all fission products and actinides, not just representative elements from each group. The effect of radiolysis will also be studied. Comparable data will be generated on unirradiated waste-form samples. Both sets will be used to qualify the waste for disposal in a geological repository.
Almost as important as the waste-form characteristics, data will also be gathered on the operation of waste-processing equipment in a remote hot cell environment. Results from the demonstration should provide enough data about the process and waste forms to make knowledgeable decisions about applying electrometallurgical technology to the treatment and stabilization of other spent nuclear fuels.
Assuming that the results from the demonstration-scale test with irradiated materials are favorable, very few issues will remain to be answered before applying the process on a production scale. At production scale, the rate of fuel treatment will be higher. The waste-form production equipment will need to be capable of supporting the higher rates. For the ceramic waste, the HIP may require further scale-up. For the metal waste, scale-up may also be required for casting operations. In each case, a head start on the parameters for design and operating this new equipment should be available from the development work.
The main area requiring further testing beyond the demonstration involves the long-term durability of the waste forms. Much of the waste-form work with irradiated materials will not occur until the final months of the demonstration. Initial results from performance tests will be available, but long-term tests with samples from irradiated materials will still be ongoing. The data available after the demonstration will be enough to provide a reasonable assurance that the waste forms are acceptable, but long-term testing will be required to qualify them for final disposal.
The PHP is a high-temperature, thermal process developed for the treatment of mixed wastes. The process produces a reduced-volume, highly stable, vitreous product. Mixed wastes consist of sludge and miscellaneous debris that are lightly contaminated with radionuclides. This waste has been generated throughout the DOE complex for decades, packed into drums or boxes, and stored above ground or buried in shallow pits. An attractive feature of the PHP is that intact containers can be fed to the process without the need for opening, sorting, or sizing, thereby potentially reducing handling requirements, costs, secondary wastes, and worker exposures. Typical feed to a production-size PHP would be 208 liter drums, in which much of the accumulated mixed waste is stored.
The PHP benchscale project features a reduced-scale system, fully configured for operation with radioactive waste materials. This system complements nonradioactive PHP systems that have been constructed and tested elsewhere using surrogate wastes. The benchscale project will determine the behavior and fate of radionuclides for comparison with the surrogate test results and assess the performance of the PHP from a broader perspective than has been done.
Metals, ash, and inert materials that do not volatilize, but remain in the plasma chamber, are melted and produce a molten pool of metal and various oxidized materials that form a slag. The molten material is captured in a shallow, refractory-lined hearth, from which it would be periodically removed by a drain or pour spout to canisters for placement in a waste repository. The pool tends to separate by gravity into a molten-metal layer underneath the molten slag. By limiting the quantity of air that is allowed into the plasma chamber, the molten metal can largely be kept from oxidizing. The benchscale system does not currently have a means for draining the molten materials. Rather, the system is operated in a batch mode, processing up to nine 4 l containers of waste (scaled-down 208 l drums) and retaining all molten materials in the hearth where it solidifies after shutdown of the system. Following cool-down, the plasma chamber is opened, and the solidified residues are extracted from the hearth.
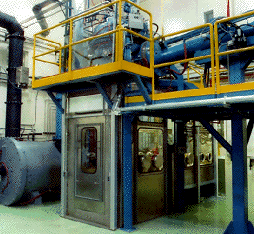 |
| Figure 5. The PHP benchscale test facility. |
Long-term durability determinations are less defined, but typically include the Product Consistency Test (PCT),14 which is similar to the TCLP but may reveal more about the waste form following a longer exposure. Other parameters, such as compressive strength, may be relevant to specific waste forms. The DOE mixed wastes typically have a sufficient quantity of glass-forming materials (e.g., sand) to produce a glass-like slag in the PHP. These vitreous slags have been shown to have excellent waste-retention and durability characteristics.
The most important secondary-waste minimization issue for the PHP is the distribution of the radionuclides throughout the process equipment. Earlier surrogate testing found that the surrogate material (cerium has typically been used to represent plutonium and uranium) was oxidized and migrated to the slag. Similarly, high-vapor-pressure hazardous metals have also been reported to oxidize and partition to the slag.15 Partitioning of the radionuclides to the off-gas components is generally undesirable, since this creates a new mixed-waste stream that requires further treatment. Substantiation of these results and comparative data with actual radioactive materials is vitally needed to complete the evaluation.
The PHP benchscale system at ANL-W has provisions for alpha-contamination controls, remote operation of the system, full radiological monitoring of the process, and the extensive sampling and laboratory-analysis program required for radioactive operations. The PHP facility is also provided with extensive, state-of-the-art instrumentation on the off-gas system to yield a more complete understanding of the operation.
The experimental program is designed to determine the major and minor effects of processing feed materials in terms of chlorine content, organic-carbon content, iron content, alkali and alkaline-earth content, and silica/alumina/magnesia content on actinide partitioning, formation and volatilization of plutonium compounds, and actinide entrainment in the off-gas. Comparative data using cerium along with plutonium, uranium, and other actinide metals will be obtained. Sample locations include the solidified slag, metal, HEPA filters, acid-gas scrubber liquor, and several collection points for liquid condensate. In addition, there are deposition coupons that are located in the off-gas ducts upstream of the filters, and refractory plugs from the plasma chamber. Off-gas measurements16 include EPA-sanctioned multiple-metals extractions; several stations of in-situ Fourier transform interferometry (FTIR) measurements of CO2, CO, NOX, HCl, and SOX; extractive FTIR measurements at several points for volatile organic vapors; laser-induced breakdown spectroscopy measurements for metal vapors; two-color laser transmissometry measurements of particulate size and number density; and pyrometry temperature measurements in the plasma chamber.
Construction and checkout testing of the PHP facility and equipment were completed in the fall of 1996. Since that time, seven nonradioactive experiments have been conducted. Preparations are underway to begin the radioactive experiment program in summer 1997.17
Waste recipes for these experiments have been deduced from published reviews and manifests of the contents of the extensive mixed-waste inventory within the DOE complex. The wastes fall into the broad categories of sludge and debris. Variations within the sludge category range from organic to inorganic, and within the debris category from combustible to noncombustible. The nonradioactive experiments that have been conducted in the benchscale system thus far consisted of five inorganic-sludge, one mixed-debris, and one highly combustible debris. Recipes that mimic these wastes were selected and prepared with spikes of carefully measured quantities of hazardous heavy metals, cerium, and a stable cesium-chloride compound. Typical recipes for the test containers are shown in Table I with results from early tests in the benchscale PHP demonstration. One experiment included relatively large blocks of wood, graphite, and refractory to determine if they could be readily processed in the PHP. These materials have been found to create processing problems in other thermal-treatment systems.
| Table I. Typical Heterogeneous Debris and Inorganic Sludge Tests | ||
|---|---|---|
| Process Feed Material | ||
| Typical Heterogeneous Debris | Percent | Results and Observations |
| Aluminum | 1.0 | High-iron debris, blocks of wood, and graphite were readily processed. |
| Aqueous Organics | 2.0 | |
| Cardboard | 1.0 | |
| Alumina Ceramics | 1.0 | Blocks of high-alumina refractory did not process well, due to inability to concentrate the plasma on the block. This implies that there may be a potential for blockage of molten material drains or pour spouts, which should be further investigated. |
| Concrete | 9.8 | |
| Soil | 3.9 | |
| Glass | 16.6 | |
| Oil-Dry Absorbent | 1.0 | |
| Paper | 2.0 | |
| Polyethylene | 2.9 | |
| Polyvinyl Chloride | 1.0 | |
| Rubber | 2.0 | |
| Gypsum Board | 7.8 | |
| Carbon Steel | 42.0 | |
| Wood | 3.9 | |
| Spikes (total) | 2.3 | |
| Hazardous Metals | Cesium and the hazardous metals, with the exception of barium, were largely volatized to the off-gas system. TCLP tests showed the leachate to be below the threshold, but few hazardous metals were in the slag to start with. The cerium appeared to be completely contained within the slag, with no perceptible distribution to the off-gas system. | |
| Arsenic Trioxide | 0.13 | |
| Silver | 0.10 | |
| Mercury Sulfide | 0.12 | |
| Barium Oxide | 0.11 | |
| Selenium | 0.10 | |
| Lead | 1.00 | |
| Cesium Chloride | 0.36 | |
| Cerium Oxide (Pu surrogate) | 0.34 | |
| Typical Inorganic Sludge | Percent | Results and Observations |
| Aluminum Oxide | 1.0 | The inorganic sludge was readily processed |
| Calcium Sulfate | 4.9 | |
| Diatomaceous Earth | 8.9 | |
| Hydrated Lime | 9.9 | |
| Iron Oxide | 7.9 | |
| Magnesium Oxide | 4.9 | |
| Portland Cement | 7.9 | |
| Sodium Nitrate | 13.8 | |
| Water | 39.4 | |
| Spikes (total) | 1.5 | |
| Hazardous Metals | Cesium and the hazardous metals, with the exception of barium, were largely volatized to the off-gas system. TCLP tests showed the leachate to be below the threshold, but few hazardous metals were in the slag to start with. The cerium appeared to be completely contained within the slag, with no perceptible distribution to the off-gas system. | |
| Cadmium Oxide | 0.11 | |
| Chromium Oxide | 0.15 | |
| Nickel Oxide | 0.13 | |
| Lead Oxide | 0.11 | |
| Mercury Sulfide | 0.12 | |
| Barium Oxide | 0.11 | |
| Beryllium | 0.10 | |
| Cesium Chloride | 0.38 | |
| Cerium Oxide (Pu surrogate) | 0.19 | |
| Volatile Organics | ||
| Carbon Tetrachloride | 0.10 | The volatile organics were destroyed. |
Future experiments will determine partitioning using actual radionuclides, further explore the conditions affecting the final waste-form properties, and study the volatilization of the hazardous heavy metals. Experiments will be conducted to determine if HEPA filters that capture all of the particulate, including most condensed heavy metals, can be recycled effectively back into the process. Destruction of hazardous organic material will be investigated, as will active, feedback control approaches for maintaining the slag product within specified bounds.
Because of their versatility and low cost, cementitious materials have been extensively studied for encapsulating low- and intermediate-level nuclear waste.18-23 These applications, however, have met with limited success, because typical cementitious materials do not always meet performance requirements for structural integrity and leach resistance. Recent developments with material formulations and process techniques are enabling the production of a new class of stronger cements that are more suitable for the task of nuclear-waste isolation. Advances in cement compositions and the processes for hydration, consolidation, and densification have produced about a tenfold enhancement of such waste-form properties as compressive strength, bending strength, and leach resistance. The performance of these new cements is comparable in many respects to conventional ceramics that have been formed by high-temperature sintering or fusion.24
These new materials are a class of chemically bonded ceramics that are manufactured at lower temperatures like typical hydraulic cements, but perform much more like high-temperature fused ceramics.25 The name derives from the low-temperature reduction/oxidation reactions by which the material is bound. These materials are formed by a combination of ionic, covalent, and van der Waals bonding, with the stronger ionic and covalent bonds dominating. Chemically bonded ceramics are much stronger than typical room-temperature hydraulic cements that are formed by comparatively weaker van der Waals and hydrogen bonding.
Recent attention has been focused on CBPCs,26 which have been known for some time to be amenable to the low-temperature formation of strong ceramic materials.27 The reduced solubility of metallic-phosphate compounds, which has long been understood, has been used to reduce metal leach rates from wastes.28,29 By design, the compositions of artificial CBPCs replicate naturally occurring phosphate minerals. These minerals (e.g., monazites, autunites, and apatite derivatives) have served as host matrices for naturally radioactive elements such as uranium and thorium over geologic time periods.
Some stakeholders have objected to thermal nuclear-waste treatment processes because of the potential for the emission of hazardous or radioactive materials into the atmosphere30 and a general public distrust of incinerators. Stakeholders have also objected to immobilization of radioactive waste in grout,31 because its long-term durability is suspect at best. The DOE's interest in CBPCs in part derives from the technology's ability to overcome both of these potential problems. The non-thermal process produces no hazardous off-gases, but the ceramic product can have the mechanical properties and leach resistance of vitreous waste forms. Further, the simplicity of an aqueous slurry cementitious process is advantageous for radioactive wastes that must be handled and processed within gloveboxes or hot cells. One disadvantage is that hazardous organic materials are not destroyed as in a thermal process, resulting in a somewhat larger volume.
One example of CBPC formulation is thoroughly calcined magnesia (MgO) and potassium dihydrogen phosphate (KH2PO4) mixed with water in stoichiometric proportion to produce hydrated magnesium potassium phosphate (MKP, MgKPO4·6H2O) in a batch operation. Waste metals are introduced to the slurry complex to varying degrees to form mineral analogs, which become components of the matrix or encapsulated components within the matrix. The matrix components are mixed vigorously with up to 50 wt.% fine, granular, or powdered waste in a device such as the one shown in Figure 6 for about one-half hour to form a pourable slurry.
 |
| Figure 6. A chemically bonded phosphate ceramics mixer. |
Ceramicists at ANL initially began investigating the applicability of CBPCs to mixed waste streams with the intent of utilizing the metal-binding properties of the phosphate compounds to form solid wastes that will pass standard leach tests for characteristic hazardous metals. Laboratory-scale tests with hazardous waste are being scaled up for tests on problematic DOE mixed waste streams. Necessary process adaptations for both batch-size scale-up and remote operations in controlled atmospheres are being developed.
The process is presently being scaled-up at ANL-W for mixed-waste streams. Stabilization of mercury-contaminated crushed glass is being demonstrated to test the use of CBPCs as a hot-cell-based microencapsulation tool. Additives are being used to promote the stabilization of both mercury and the more soluble radioactive elements, including cesium. Lead-lined neoprene gloves will be stabilized by macroencapsulation in a CBPC matrix, which the EPA specifies as the appropriate treatment technology for radioactive lead solids. However, macroencapsulation alone does not remove the hazardous characteristic, which is a problem if the waste form is to be disposed as radioactive waste. In order to remove the waste from RCRA regulation, the waste form must meet the leach criteria for the characteristic hazardous metals. Scientists at ANL are investigating waste pretreatment schemes such as cryofractionation to powderize the contaminated gloves prior to CBPC stabilization. This is intended to maximize interaction between the CBPC matrix material and the metal. There are three areas of development in this demonstration: adaptation of the technology for the treatment of specific mixed waste streams, scale-up, and process optimization in glovebox and hot cell environments.
CBPC stabilization technology is also being developed and demonstrated for transuranic wastes. Scale-up, process optimization, and operations in controlled environments are also challenges for this project. Because the process places fissile plutonium in a moderated, reflected state, criticality control is a significant engineering constraint.
Additional performance criteria for actinide-bearing waste forms include standards for nuclear materials safeguards and industrial safety. Specifically, a safeguards requirement exists to stabilize plutonium-containing residues in final waste forms from which actinide recovery is difficult. To meet this standard, the process is being fine-tuned to accentuate matrix properties that enhance resistance to actinide separation from the monolith. Another issue with these wastes is control of hydrogen gas generation. Because this technology combines alpha-decaying transuranic wastes with bound water, the final waste forms may release hydrogen gas through radiolysis. If sufficient gas is generated and concentrated under conditions of storage, an industrial explosion hazard can be created.
The development of processes for producing viable final waste forms while reducing this gas-generation potential is a goal of the project. A variety of techniques, including bound water retort and precomplexing the actinides with silica, are being developed. For the demonstration, plutonium-containing incinerator residues from the Rocky Flats Environmental Technology Site32 are being stabilized and tested for actinide recoverability and gas generation.
Hot demonstrations are a necessary part of the successful development of new nuclear-waste processing technologies. The term demonstration sometimes conjures up the image of an almost pro forma test involving well-developed equipment, processes, and products. As seen in the case of the three ANL-W nuclear-waste technology demonstrations, the situation is not only more complex, but some of the nonradioactive development is actually done to support the hot demonstration. Inevitably there is some blurring of the line between the laboratory and industrial application.
The desire to bypass the hot-demonstration phase of development is easy to understand; hot demonstrations are expensive and they delay deployment, sometimes by years. Consider the three ANL-W demonstrations. The price of qualifying a high-level waste for geologic disposal is still unknown,33 but has been informally estimated to range from a low of $20 million to a high of $2 billion. But since no high-level waste has been qualified, no standards for qualification exist, and no geologic repository has been licensed, the true cost has not been established for any high-level waste form. Engineering-scale development of high-level waste forms is currently a modest, but significant, fraction of Argonne's $25 million per year electrometallurgical treatment demonstration project. The PHP demonstration, which involved facility modification and equipment procurement, has cost somewhat in excess of $10 million and taken some four years to near completion. The CBPC demonstrations, which take advantage of low-priced equipment and existing gloveboxes, will cost less that $1 million and can be completed within a year of project conception.
Even with the cost and delay in deployment, just the engineering difficulties that have to be overcome for the demonstrations are proof enough that large capital expenditures should not be risked until the processes are proven, the equipment is reliable, and the waste streams are adequately defined. Figure 7 shows damage to the PHP hearth that occurred during one of the nonradioactive tests. This problem can be readily remedied during the demonstration with relatively simple modifications, but had a full-size PHP system broken down during TRU waste processing, repairs to the complex, plutonium-contaminated equipment would have been very expensive. A recent breakdown in a simpler version of the melter technology that will be used for treating the TRU waste stored in Idaho illustrates the expense of shutdowns for repairs during actual waste processing.34
 |
| Figure 7. Hearth damage caused by plasma arc. |
Hot demonstrations should be built into the development plan at an early stage to help reduce the time required to go from concept to deployment. Many different scientific and engineering disciplines are required to develop a successful nuclear-waste treatment technology. Trying to shortcut the disciplined development cycle and jump prematurely into deployment of untested equipment and facilities is simply foolish.35 Problems should be worked out at a scale and within a program in which capital, environmental, and safety costs can all be predictably controlled. This is the essential role of hot demonstrations of nuclear-waste processing technologies.
| POST-PUBLICATION UPDATE |
|---|
|
The following letter to the editor was sent to JOM following publication of this article: Dear Mr. Robinson: I would like to make a couple of important points that were not included in our paper "Hot Demonstrations of Nuclear Waste Processing Technologies," which was published in the July 1997 JOM, pages 14-21; 83. One is a serious omission, and the other is a significant development since we submitted the manuscript. On page 20, we mentioned ceramicists at ANL who developed the processes that will be demonstrated in the chemically bonded phosphate ceramics project here at ANL-W, but we failed to include their names or to cite any reference to their work. A place-holder reference was inadvertently dropped in our haste to meet the publication deadline with the final manuscript. Our apologies go to our collaborators Arun Wagh and Dileep Singh, whose pioneering work in the application of CBPCs to nuclear waste stabilization made this project possible. The citations that we had intended to include are 1. A.S. Wagh, D. Singh, S.Y. Jeong, and R.V. Strain, "Ceramicrete: Stabilization of Low-Level Mixed Wastes—A Complete Story," Proc. 18th USDOE Low-Level Radioactive Waste Management Conf., http://www.inel.gov/resources/research/.llrw/1997Conference/. 2. A.S. Wagh, S.Y. Jeong, D. Singh, R. Strain, H. No, and J. Wescott, "Stability of Contaminated Solid and Wastewater with Chemically Bonded Phosphate Ceramics," Proc. Waste Management Annual Mtg., http://www.wmsym.org/wm97proceedings/. 3. D. Singh, V. Mandalika, A. Wagh, R. Strain, and M. Tlustochowicz, "Immobilization of 99Tc in Low-Temperature Phosphate Ceramic Waste Forms,"Proc. 99th Ann. Mtg. of the American Ceramic Society, to be published. On page 21, we mentioned some problems with the plasma hearth treatment system that would have been serious had they occurred during radioactive operation. During subsequent nonradioative testing, even more troubling malfunctions have occurred. Given the operational problems and the observed volatilization of the hazardous metals to the off-gas system, we have decided to postpone radioactive testing indefinitely. Any follow-on work would involve significant modifications to the system and, possibly, to the operating conditions.
Sincerely, |
This work was performed under contract W-1-109-Eng-38 for the U.S. Department of Energy, Office of Nuclear Energy, Science and Technology, and the Office of Environmental Management.
References
1. D.B. Black, C.C. Dwight, and M.J. Lineberry, "Use of Nuclear Facilities at Argonne-West to Support New Environmental Missions" (Paper presented at the Dixy Lee Ray Memorial Symposium on Science-Based Environmental Management, Seattle, Washington, August 30-September 2, 1994).
2. Executive Summary, The 1996 Baseline Environmental Management Report, DOE/EM-0290 (Washington, D.C.: DOE, June 1996), p. 1.
3. "Idaho, EPA Fine DOE Close to $1M for Pit 9 Cleanup Delays at INEEL," Nuclear Waste News (March 27, 1997), p. 120.
4. Environmental Assessment Electrometallurgical Treatment Research and Demonstration Project in the Fuel Conditioning Facility at Argonne National Laboratory-West, DOE/EA-1148 (Washington, D.C.: DOE, May 1996).
5. D.L. Fillmore, "Characteristics of Department of Energy Spent Nuclear Fuel," Topical Meeting on DOE Spent Nuclear Fuel Challenges and Initiatives (La Grange Park, IL: ANS, 1994), pp. 282-287.
6. W.H. Hannum, ed., Progress in Nuclear Energy, 31 (1/2) (1997).
7. "DOE Awards BNFL TEAM $1.18M Mixed Waste Treatment Contract," Nuclear Waste News (Jan. 2, 1997), p. 5.
8. G.L. Leatherman et al., Results of STAR Center Testing in Support of Non-Radioactive Pilot-Scale Plasma Hearth Process System, SAIC-95/1313 (Idaho Falls, ID: SAIC, October 1995).
9. W.P. Wolfe, R.M. Geimer, and J.A. Batdorf, Plasma Hearth Process Pilot-Scale System Title-II Design Summary, SAIC-95/1011(Idaho Falls, ID: SAIC, September 1995).
10. J.E. Battles et al., "Pyrometallurgical Processes for Recovery of Actinide Elements," International Symposium on Actinide Processing Methods and Materials (Warrendale, PA: TMS, 1994), pp. 135-148.
11. K.M. Goff et al., "Fuel Conditioning Facility Electrorefiner Start-Up Results," DOE Spent Nuclear Fuel and Fissile Material Management, (La Grange Park, IL: ANS, 1996).
12. E.C. Gay, W.E. Miller, and J.J. Laidler, "Proposed High Throughput Electrorefining Treatment for Spent N-Reactor Fuel," DOE Spent Nuclear Fuel and Fissile Material Management (La Grange Park, IL: ANS, 1996).
13. "Toxicity Characteristic Leaching Procedure, Method 1311," Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, SW-846 3rd ed. (Washington, D.C.: EPA, 1986), as amended by Updates I (July, 1992), II (September, 1994), and IIA (August, 1993).
14. "Standard Test Methods for Determining Chemical Durability of Nuclear Waste Glasses: The Product Consistency Test (PCT)," 1995 Annual Book of ASTM Standards (Philadelphia, PA: ASTM, 1995), p. 797.
15. R.L. Gillins and S.D. Poling, "Plasma Hearth Waste Treatment Demonstration for Radioactive Mixed Waste," (Paper presented at the 1994 Incineration Conference, Houston, TX, May 10, 1994).
16. K.P. Carney et al., "A Remote FTIR Monitoring of a Radioactive Off-Gas System for the Plasma Hearth Process (Paper presented at the Federation of Analytical Chemistry and Spectroscopy Societies, Kansas City, MO, October 1996).
17. R.L. Gillins et al., "A Plasma Hearth Process Bench-Scale Demonstration Project," Waste Management 97 (Tucson, AZ: W.M. Symposia, March 1997).
18. J.G. Moore et al., "Leach Behavior of Hydrofracture Grout Incorporating Radioactive Wastes," Nucl. Technol., 32 (39) (1977).
19. D.M. Roy and G.R. Gouda, "High-Level Radioactive Waste Incorporation into (Special) Cements," Nucl. Technol., 40 (214) (1978).
20. D.M. Roy, "Cement Based Nuclear Waste Solidification Forms," Conference on High-Level Radioactive Solid Waste Forms, Nuclear Regulatory Commission Conference Proceedings, no. 5, ed. L. Casey (Washington, D.C.: Nuclear Regulatory Commission, 1979), p. 479.
21. M.W. Barnes, C.A. Langston, and D.M. Roy, "Leaching of Saltstone," Scientific Basis for Nuclear Waste Management VIII, vol. 44 of Mater. Res. Soc. Symp. Proc., eds. C.M. Jantzen, J.A. Stone, and R.C. Ewing (Pittsburgh, PA: MRS, 1985), p. 865.
22. W. Fajun, M.W. Grutzeck, and D.M. Roy, "Leaching of Warm-Pressed CsZP-Cements Composite Waste Forms," Scientific Basis for Nuclear Waste Management VIII, vol. 44 of Mater. Res. Soc. Symp. Proc., eds. C.M. Jantzen, J.A. Stone, and R.C. Ewing (Pittsburgh, PA: MRS, 1985), p. 899.
23. D.M. Roy et al., "A Low Temperature Ceramic Waste Form," International Symposium of Ceramics in Nuclear Waste Management, eds. T.D. Chikalia and J.M. Mendel (Columbus, OH: American Ceramic Society, 1979), p. 136.
24. D.D. Double, "Chemically Bonded Ceramics: Taking the Heat Out of Making Ceramics," J. for Mater. Educ., 12 (1990).
25. D.M. Roy, "New Strong Cement Materials: Chemically Bonded Ceramics," Science, 235 (1987).
26. A.K. Sakar, "Phosphate Cement-Based Fast-Setting Binders," Ceram. Bull., 69 (2) (1990).
27. W.D. Kingery, "Fundamental Studies of Phosphate Bonding in Refractories: II. Cold-Bonding Properties," J. of the Amer. Ceram. Soc., 33 (1950), pp. 242-247.
28. "Treatment and Use: The Future of Ash Management," Solid Waste and Power, 5 (5) (1991).
29. L.A. Boatner and B.C. Sales, "Monazite," Radioactive Waste Forms for the Future, eds. W. Lutze and R.C. Ewing (New York: North-Holland Physics Publishers, 1988).
30. Report on the U.S. EPA Technical Workshop on WTI Incinerator Risk Assessment Issues, EPA/630/R-96/001 (Washington, D.C.: EPA, May 1996).
31. "DOE Signs New Hanford Cleanup Pact with State," The Energy Daily, 22 (16) (January 26, 1994), p. 2.
32. Ash Residues End State Trade Study, DOE/ID-10560 (Washington, D.C.: DOE, 1996).
33. Spent Nuclear Fuel Management Cost Evaluation Report, DOE/SNF/FEP-PS-001 (Washington, D.C.: DOE, March 1995).
34. "GTS Duratek Shuts Down SRS Melter," Defense Cleanup (April 4, 1997), pp. 3-4.
35. R.A. Langly, "The BNFL, Inc. Approach to Privatization," Waste Management 97 (Tuscon, AZ: W.M. Symposia, 1997).
K.M. Goff earned his Ph.D. in nuclear engineering at Georgia Institute of Technology in 1991. He is currently group leader of waste process technology at Argonne National Laboratory.
F.S. Felicione earned his Ph.D. in thermal sciences at the University of California at Berkeley in 1970. He is currently project manager of the Plasma Hearth Process Project at Argonne National Laboratory.
C.C. Dwight earned her B.S. in nuclear engineering at Idaho State University in 1985. She is currently group leader of waste programs in the Technology Development Division at Argonne National Laboratory.
D.B. Barber earned his M.S. in health physics at Colorado State University in 1993. He is currently a staff engineer at Argonne National Laboratory.
For more information, contact H.F. McFarlane, Argonne National Laboratory-West, P.O. Box 2528, Idaho Falls, Idaho 83403-2528; (208) 533-7106; fax (208) 533-7735; e-mail harold.mcfarlane@anl.gov.
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|