 |
and is presented as JOM-e. Such articles appear exclusively on the web and do not have print equivalents. |
|---|
 |
and is presented as JOM-e. Such articles appear exclusively on the web and do not have print equivalents. |
|---|
 |
|---|
| CONTENTS |
|
|
Several studies regarding the kinetic aspects of calcium-iron silicates1 and the effect of CO/CO2 mixtures and sulfur content on calciowustite have been reported.2,3 In the systems CaO-FeO-Fe2O34-6 and CaO-FeOx-SiO2,7 most of the data are for iron-making temperatures, but few studies relate to nonferrous processing temperatures. Takeda et al.8 provided basic information on the activity of components at 1,473 K and 1,573 K for the CaO-FeO-Fe2O3 system, while the liquidus isotherms were investigated by Schumman et al.9 Furthermore, the phase relations of the CaO-Fe2O3-SiO2 and CaO-FeO-SiO2 systems under air atmosphere and in equilibrium with gamma iron have been reported.10-12
copper concentrates contain significant levels of siliceous components. To successfully use lime flux, the dissolution of silica in calcium-ferrite slags must be determined. By adding different levels of silica to a CaO-FeOx slag, the liquidus isotherm lines at 1,473-1,573 K under iron saturation and air and other atmospheres were investigated.
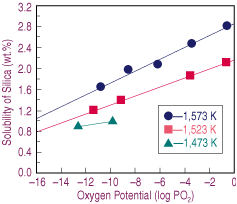 |
| Figure 1. The effect of oxygen potential on the solubility of silica in calcium-ferrite slags melted at different temperatures. |
Equilibrium smelting tests were carried out at a fixed initial composition, temperature, and atmosphere with slags containing several silica contents. Once equilibrium was attained, the samples were rapidly solidified, and their upper and lower halves analyzed for silica. Any compositional difference was attributed to the presence of a primary solid, which was later confirmed by microscopic observations. The solubility limit for silica was estimated as the value where no difference in composition between the upper and lower halves of the sample was determined, as proposed by Takeda et al.8 The samples were chemically analyzed by standard methods for calcium, silica, and ferrous and total iron when no second phases were observed via optical microscopy.
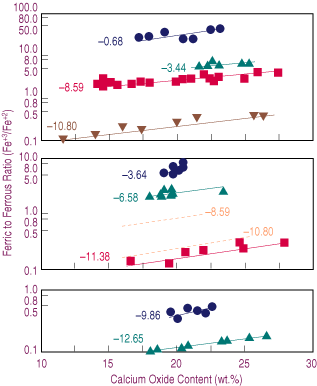 |
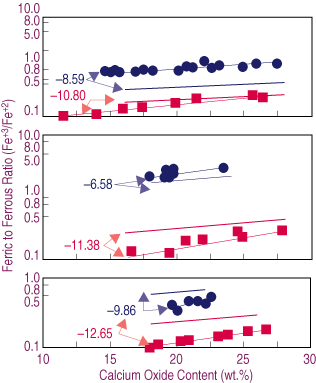
| Figure 2. The effect of lime content on the oxidation degree (Fe+3/Fe+2) of the slag for samples melted at different temperatures and oxygen potentials (logPO2)—(a) 1,573 K, (b) 1,523 K, (c) 1,473 K. Dashed lines represent estimated behavior. |
The effect of the oxygen potential upon the oxidation degree of the slag can be realized, at a first stage, by considering the equilibrium wustite-hematite, according to
| FeO(s) + 1/4 O2(g) = FeO1.5(s) | (1) |
By replacing the appropriate thermodynamic data,14 the following is obtained.
| log(aFeO1.5/aFeO) = 1/4 logPO2 - (7441.14/T-2.57) | (2) |
which, in terms of the ferric-to-ferrous ratio (assuming Henrian behavior), can be written as:
| log(Fe+3/Fe+2) = log(YFeO/YFeO1.5) + 1/4 logPO2 - (7441.14/T - 2.57) | (3) |
The effect of temperature upon the oxidation degree can be explained by Equation 2. A decrease in temperature results in the second term on the right side of the equation becoming larger. If the oxygen potential and the ratio of activity coefficients in Equation 3 are assumed constants, the result is a decrease in ferric-to-ferrous ratio, as observed from Equation 3 in agreement with the data presented in Figure 2.
In Figure 2b, the estimated Fe+3/Fe+2 ratios corresponding to oxygen potentials of 10-8.59 and 10-10.80 at 1,523 K are presented as thick dashed lines. This range of oxygen potentials was selected because the slags show the wider range in calcium content. A comparison of the data in Figure 2a and Figure 2b shows that a decrease in temperature results in a lower Fe+3/Fe+2 ratio at a constant oxygen potential.
Although calcium ferrites are the main compounds formed, the following equilibria may also be considered.
| 2 CaO(s) + Fe2O3(s) = Ca2Fe2O5(s) | (4) |
| CaO(s) + Fe2O3(s) = CaFe2O4(s) | (5) |
| CaO(s) + 2 Fe2O3(s) = CaFe4O7(s) | (6) |
At low oxygen potentials under the coexistence of lime, wustite, and calcium ferrite, Reaction 5 may be written as
| CaO(s) + 2 FeO(s) + 1/2 O2(g) = CaFe2O4(s) | (7) |
| Table I. Equilibrium Constants for Calcium Ferrites and Dicalcium Silicate Formation | ||
|---|---|---|
| Reaction | Temperature (K) | Equilibrium Constant (Keq) |
| CaO(s) + 2 FeO(s) + 1/2 O2(g) = CaFe2O4(s) | 1,573 | 2.24x104 |
| CaO(s) + 2 FeO(s) + 1/2 O2(g) = CaFe2O4(s) | 1,573 | 4.93x104 |
| 2 CaO(s) + Si2(s) = Ca2SiO4(s) | 1,573 | 2.87x104 |
| 2 CaO(s) + Si2(s) = Ca2SiO4(s) | 1,573 | 3.94x104 |
The equilibrium constants for calcium ferrites and dicalcium silicate formation are given in Table I;13 the tendency to form monocalcium ferrite is as strong as the tendency to form dicalcium silicate. It has been reported that reaction products such as calcium ferrites decrease the tendency to form dicalcium silicate,14 and the interaction between lime and iron oxides becomes stronger with increasing oxygen partial pressures.15 In fact, under these conditions, monocalcium ferrite (CaFe2O4) was always detected.16
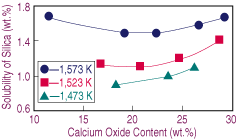 |
| Figure 3. The effect of lime on the solubility of silica in calcium-ferrite slags melted under iron saturation. |
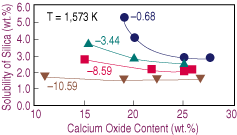 |
| Figure 4. The effect of lime on the solubility of silica in calcium-ferrite slags melted at 1,573 K under different oxygen potentials (logPO2). |
As the oxygen potential increases, the nose in the FeOx-CaO-SiO2 is wider and shifted toward the CaO corner.17 Therefore, as the slag dissolves, the change in silica solubility with lime content becomes steeper. Nevertheless, there is experimental evidence for the opposite arguments: the position of the nose depends on the oxygen potential and is extended away from the CaO corner toward lower silica contents.16 In view of this situation, further research on the effect of oxygen potential and temperature upon the solubility of silica at low lime contents is advisable.
Increasing the equilibrium temperature results in higher solubilities of silica. Furthermore, a decrease in temperature stretches the range at which calcium oxide melted. Results obtained at 1,573 K for equilibrium tests under different oxygen potentials are presented in Figure 4, which shows similar behavior to the test under iron saturation, with the exceptions of the narrower range of lime at which the silica level is constant and the faster change in solubility toward low lime contents.
 |
| Figure 5. A comparison of the experimental results and Takeda's Equation 8 (denoted by dashed lines)—(a) 1,573 K, (b) 1,523 K, (c) 1,473 K. |
An approach to realize the effect of lime upon the solubility of silica is provided by Takeda et al.,8 who relate the oxidation degree of the slag with its lime content. Although their analysis is based on the plain CaO-FeOx system, it can be extrapolated to CaO-FeOx-SiO2 due to the low solubility of silica. Takeda et al. relate the Fe+3/Fe+2 ratio with the CaO content in the slag under iron saturation up to oxygen potentials of -4 through
| log(Fe+3/Fe+2) = 0.170·logPO2 + 0.018·(%CaO) + 5500/T - 2.52 | (8) |
With this equation, it is possible to estimate the effect of oxygen potential, temperature, and lime content upon the ferric-to-ferrous iron ratio. The present results and those evaluated through Equation 8 are presented in Figure 5. At the lowest oxygen potentials, silica decreases the ferric-to-ferrous ratio in agreement with the behavior shown in the FeO-Fe2O3-SiO2 system; however, as the oxygen potential increases, so does the ferric-to-ferrous ratio. These results suggest that there is a compromise between lime and silica in respect to ferric and ferrous iron. For example, in Figure 5a and Figure 5b, the results at the lowest oxygen potentials when compared to Takeda's show lower ferric-to-ferrous ratios. However, at higher oxygen potentials, the opposite behavior is observed, except at 1,473 K.
Akira Yazawa earned his Dr.Eng. in extractive metallurgy at Tohoku University, Japan, in 1953. He is currently emeritus professor at Tohoku University and is an international consultant.
For more information, contact C.M. Acuña, Codelco Chile, Chuquicamata Smelter R&D/Surgerencia Fundición de Concentrado, Chuquicamata—Chile; telephone (055) 325437; fax (055) 323488; e-mail acuna@chuq.codelco.cl.
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|