 |
51 (5) (1999), pp. 24–28. |
|---|
 |
51 (5) (1999), pp. 24–28. |
|---|
 |
| TABLE OF CONTENTS |
|---|
Earlier analysis and discussions of alternative processing1 demonstrated that the fundamental energy requirements for all options do not differ substantially since all start with an aluminous (oxide) raw material and all finish with aluminum at a temperature above its smelting point, with oxygen and carbon oxide by-products. From a theoretical energy consideration, different processes do not hold much potential to make substantial differences, although there can be a trade-off between electricity and carbon as the energy source used. Thus, from an energy perspective, the differences in processes usually revolve around differences in efficiencies of both the reactions unsolved in the process and the energy utilization. Some of the alternative processes considered and investigated have been based on an incomplete analysis of the process energy requirements, while others have not considered either economics or practicality.
Monopolar electrochemical cells are always capital intensive because the reaction rates are low per unit reaction area and also low per unit reactor volume. These cells have a limited finite size based on feeding technology and the need to have a liquid-metal cathode. Thus, if there is to be a significant breakthrough in the capital cost barrier, electrode arrangements and design need to be considered so that there is an increase in reactor area per unit volume. Another contributor to the capital cost is the limited cell life through corrosion and erosion of materials. Although cell lives have increased, so too has the cost of the better materials of construction.
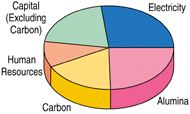
Whenever alumina is used in the presence of fluorides, as seen in Figure 1, the capital cost of the smelters is a significant contributor to the overall metal production cost. Typically, equipment for environmental compliance contributes about ten percent of this cost while extensive occupational health and compliance monitoring programs are also significant contributors to the production costs. Fluoride emissions are generated at the cell because of hydrolysis reactions. Thus, environmentally, smelters must get away from either the moisture-bearing oxide or the fluoride solvent. Alternatively, cell redesign for the much more efficient capture of all cell emissions may help costs. Candidate non-oxide feed materials have been anhydrous aluminum chloride and aluminum sulfide.
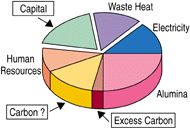
The second environmental driver is linked to the carbon-oxide greenhouse emissions. On a tonnage basis, the amount of carbon oxide produced is greater than a tonnage of metal produced. However, the only alternative to carbon oxide is electricity, since a form of energy is always required for metal production. With incremental electrical energy usually being generated by inefficient combustion processes, the potential for reducing the greenhouse impact depends on how electricity generation is accounted.
There is much more to alternative processes than simply getting the chemistry right or lowering the energy demands for any process. Four key aspects for any alternative process are
 |
| a |
 |
| b |
| Figure 1. (a) Contributions to the cost of aluminum production and (b) potential areas for cost reduction with alternative technology. |
By the mid-1980s, key engineering design features of the new breed of even larger cells included point feeders discharging less than 2 kg Al2O3 per addition; single- or dual-draft hooded emissions collection systems; a microprocessor-based process control system that takes readings of cell-energy parameters every five seconds or less and utilizes feeding and energy-change strategy according to predetermined logic; and, typically, an automated conveying system for introducing the alumina to the hoppers at the cell for a more dust-free environment.
As a consequence of further gains in process control, the trend in the 1990s has shifted further toward increasing the amperage3 or size of the cells because of the economic gains through reduced capital expenditure per unit annual production. The larger cells (especially when exceeding 310,000 A) do not achieve the low energy consumption of the slightly smaller cells of the early 1980s. This is as a result of a number of issues, including operation at higher (cathode) current density; the existence of concentrated gradients through less efficient mixing end-to-end in the cell; reduced cell life because of the more stringent heat balance requirements for freeze protection and the increased cathode corrosion with current density; and the tendency to form sludge because of the higher feed frequency and limited mixing. Despite these limitations, the overall economics are more favored through the reduction of the capital cost section of Figure 1 than through the increase in the energy-cost sector.
In reviewing all new technology and developments since the early 1980s, the following features stand out:
Based on these features, as illustrated in Figure 1b, it is evident that the greatest scope for reducing metal production costs is to reduce capital cost, have a reactor design that can operate with a lower heat loss per unit production, or eliminate the anode carbon and substitute a more cost-efficient option. This shifts the future emphasis to operable reactor designs with a low capital cost per annual tonne while complying with environmental requirements. Hence, it becomes understandable why increasing line current has become favored. This increases productivity at near constant heat loss but, for an installed technology, it is invariably less energy efficient.
Based on these general features, the best process would be one that occurs at as low a temperature as possible, but with the highest productivity per unit volume while possessing the fewest number of processing stages in the overall conversion process. In the last 25 years, there has only been sustained R&D on the first two of five discussed alternative processes (Table I).
| Table I: Alternative Processes Investigated for Aluminum Production | |
|---|---|
| Production Process | Features |
| Drained-Cell Technology* | Cathode sloping and coated with aluminum-wettable TiB2. By eliminating metal pad, anode-cathode gap could be halved to ~25 mm, enabling substantial voltage lowering. Other basics would remain the same as present technology (E° ~ 1.2 volts,  min, electrolysis = 6.34 kWh/kg). min, electrolysis = 6.34 kWh/kg). |
| Inert Anode Cells* (Oxygen Evolution) |
Eliminate consumable carbon anode by having an electrode material that evolves oxygen. Although the electrochemical potential would increase by 1 V (E° ~ 2.2 volts), the voltage increase would be (hopefully) less because of lower anode polarization ( min,electrolysis=9.26 kWh/kg). The superstructure of the existing cell could be refined, reducing capital costs. If drained-cell materials development were successful, further design options are possible. min,electrolysis=9.26 kWh/kg). The superstructure of the existing cell could be refined, reducing capital costs. If drained-cell materials development were successful, further design options are possible. |
| Chloride Process† | Aluminous material converted to (anhydrous) AlCl3 of adequate purity. AlCl3 electrochemically decomposed in a multi-electrode cell at ~700°C (E° ~ 1.8 volts,  min, electrolysis = 6.34 kWh/kg). Electrochemically generated chlorine is recycled. min, electrolysis = 6.34 kWh/kg). Electrochemically generated chlorine is recycled. |
| Sulfide Process† | Aluminous material converted to (anhydrous) Al2S3 of adequate purity. Aluminum sulfide electrochemically decomposed to recyclable S2 and aluminum (E° ~ 1.0 V) in a multipolar ( min,electrolysis = 5.24 kWh/kg) cell. min,electrolysis = 5.24 kWh/kg) cell. |
| Carbothermal Reduction† | Convert aluminous material to an intermediate Al4C3 (or oxycarbide) chemically at T > 1,700°C. React carbide with further oxide to evolve CO and produce aluminum (or alloy) at T > 2,000°C. Refine the metal quality to a usable grade ( minimum = 9.0 kWh/kg). minimum = 9.0 kWh/kg). |
| * Substantial retrofits using cryolite-alumina electrolytes. † Processing using intermediates derived from alumina. |
|
Because of its refractory nature, high melting point, and the high cost of the raw materials from which TiB2 is produced, there have been significant economic and practical challenges to fully testing and implementing wetted cells. In order to reduce the costs and fabrication difficulties for TiB2, considered approaches have included bonding tiles and other shapes to the carbon substrate8 or alternatively applying a composite coating.9,10 In the last two decades, the main driver has been to develop drained cells that, by elimination of the metal pad and, hence, its magnetically induced turbulence, enable cells to be operated at a much reduced interelectrode distance. This would lower the energy consumption through the lower voltage. However, the external design and features of the cell would essentially be the same, and, therefore, a reduction in voltage must be accompanied by a reduction in heat loss.
Theoretically, increasing the thickness of the crust cover and reducing drafting at the top of the cell would lower the heat loss. However, with increasing the thickness of the top cover, the crust becomes softer and risks collapsing. Likewise, reducing the drafting velocity leads to a greater rate of release of emissions to the environment. Therefore, the reductions in top heat loss are extremely constrained. The bottom heat loss of the cell has generally already been reduced as much as possible with modern insulation materials and is a small contributor to the total.
Insulating the sides of the cell, which typically account for a quarter of the heat loss, results in a loss of the protective sidefreeze because of the consequential increase in superheat. The importance of retaining this has already been noted. Practically, it is of even greater importance for TiB2-coated cells because of the accentuated corrosion that would occur at the three-phase interface between the liquid bath, carbon, and aluminum-wetted coating.
Drained cells have other design and operating challenges that must be met before implementations, including
Design innovation and hard slog R&D can answer these questions satisfactorily, but inevitably solutions will have a price tag that detracts from the perceived economics.
The coating material itself must also satisfy several criteria. First, its thickness must be sufficient to give the desired cell life after recognizing the metal it dissolves to saturation in the metal. Second, any binding phase of the composite must corrode at a similar rate to the TiB2. Third, the coefficient of thermal expansion should match that of the substrate to which it is bonded. Finally, the material must be impervious to the electrolyte (or electrolyte uptake totally prevented), since the electrolyte can lead to galvanic corrosion if it is also in contact with a carbonaceous substrate.
With the existing cathode potential gradient, the formation of Al4C3 becomes enhanced by the thermodynamically favored galvanic corrosion reaction within any coating material that has also taken up electrolyte. With the aluminum-wetted cathode surface being at a more anodic potential, and there being a continuing supply of aluminum and carbon, we have
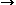 4 Al3+ + 12e
4 Al3+ + 12ein the zone above the electrolyte that has penetrated the coating, and
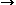 3C4-
3C4-at the carbon surface with the consequential reaction in the electrolyte phase
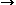 Al4C3 (solid)
Al4C3 (solid)The growth of this deposit can lead to debonding and accelerated coating failure.
Three separate approaches have been used for developing coatings—a colloidal alumina-bonded TiB2 composite (TinorTM and ThicknorTM) developed by Moltech,9 a plasma-sprayed TiB2 coating developed by SGL Carbon,11 and a carbon-bonded TiB2 composite developed by Comalco.10 Plant trials of the Moltech coatings have been conducted with positive indications of enhanced cell performance, especially in reducing the sodium uptake.12 While there have been no performance data published for the plasma coating, more extensive details have recently been revealed for the Comalco coating.10
 |
| Figure 2. Reductions in energy consumption achieved by Comalco10 using drained cathodes in existing technology. |
 |
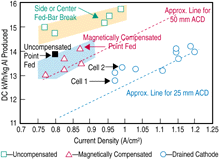
| Figure 3. The parallel development paths followed by Comalco10 in developing coated drained technology. |
When increasing the line current, the gains are lowered by consequential issues, such as cathode voltage drop increases, higher anode stub drops, and the resistivity losses of the interconnecting busbars. Perhaps equally revealing is that they found it necessary to develop not only the coating to meet the specifications, but also the cell design and operating strategy (Figure 3).
The data presented,10 together with other publications on the performance of Tinor,12 demonstrate that while the era of wetted-cathode technology is now a practical option, the economic and production gains are not likely to be dramatic unless the design and heat-balance constraints can be overcome.
 4 Al + 3O2
4 Al + 3O2would require an electrochemical voltage of approximately 2.2 V as compared to 1.2 V when using a conventional carbon anode. While it has been argued that the anode polarization will be less and will, thus, lower the necessary voltage increase, this feature can only be beneficial if the heat-balance constraint can be overcome. This means that electrical-energy reductions will not be achieved unless a major design change is also implemented. The increased power cost would, however, be partly offset by simpler operations and elimination of the carbon-anode costs.
Hitherto, no suitable anode material has been found as technical difficulties arose through corrosion and subsequent metal contamination (at a time when the industry is shifting to more high-purity metal applications); the loss of electronic conduction of the oxide surface, causing passivation and high voltages; and problems of adhesion of the oxide surface coating to the metal substrate when manufacturing at the necessary electrode size. At best, it appears the electrode design will necessitate the ability to periodically remove the electrode from the cell for surface refurbishment in a similar manner to the practice for the chlor-alkali industry. This, coupled with the challenges for heat insulation (especially to reduce top-heat losses) and alumina feeding, also emphasizes that design and operating challenges must be addressed.
The considered two-stage reduction process first forms a carbide (T > 1,900°C)
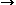 Al4C3 + 6CO
Al4C3 + 6CO(although, the presence of excess Al2O3 could also lead to an oxycarbide phase) and then react the carbide with more oxide (at T > 2,000°C)
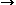 6Al + 3CO
6Al + 3COThe staging of the reactions reduces potential dusting problems through the high gas volumes otherwise released. However, it necessitates accurate temperature control for two reaction sequences in an environment that is difficult to control. Furthermore, the viscosity-composition-temperature relationships for the reacting charges present enormous challenges for operations and design.
 |
 |
| a | b |
| Figure 4. A schematic of the key features of a horizontally oriented multiple bipolar cell showing (a) the desired current path and (b) the by-pass current paths. | |
When writing the sequence of reactions, it is interesting to note that, like the carbothermal option, carbon is involved as a significant material in all of these processes (except the oxygen-evolving cell). Consequently, they will all have similar overall theoretical energy efficiencies. Differences will revolve on the varying proportions of chemical versus electrical energy input and varying reaction efficiencies.
For any of these three options to be successful, inert anodes are required. Other requirements include an operating temperature above 700°C to form liquid aluminum; an inert drained cathode; an anode that is unreactive to the anode products (e.g., chlorine, sulfide or oxygen); a corrosive electrolyte, because of the need to have it in an ionic form at elevated temperatures; a design that enables the efficient separation of the liquid aluminum product from the gaseous anode products; and energy efficiencies that reduce heat loss per unit production. Not only in aluminum smelting, but in all other metallurgical and high-temperature processes, the best reactor protection is a layer of frozen reacting mixture, and if the design does not achieve this, there is another challenge.
Multipolar electrodes such as the multiple bipolar design patented for the ASP4 have the obvious advantage that higher productivities per unit reactor volumes are possible. However, these cannot operate at the high current efficiencies currently experienced because of by-pass currents that are inevitably present. Figure 4 schematically illustrates a multiple bipolar cell that has near horizontally oriented electrodes together with the preferred current flow path. The electrolyte in both the cavity that enables the gas to be released (Figure 4b) and in the cavity where the metal drains (Figure 4a) presents an alternative path for by-pass current flow. The equivalent circuit is complex, and the design challenges to obtain high-current efficiencies are considerable. Beck, Rouser, and Thonstad17 have compared these with the inefficient monopolar arrangement and shown energy efficiencies are likely to be the same as a consequence.
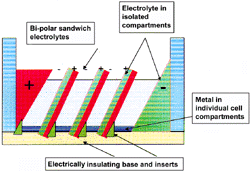 |
| Figure 5. A schematic of a compartmentalized vertically oriented multiple bipolar cell highlighting the necessary design features. |
The economic gains of alternatives are, however, not likely to be dramatic. The development costs when considering the above facets are considerable—so much so that it is impractical for any one company to do it alone. Thus, it will necessitate consortiums "picking a winner," unless there is a change in driving force from economics alone.
The challenge becomes even harder when it is recognized that there are several viable paths open to improve the existing process, so any new technology is faced with a moving target. When picking a technology, it is crucial that the concept be developed in its entirety with parallel effort on design, operating practices, materials, and reaction concepts as otherwise the development time could extend to half a century.
Titanium-diboride cathode technology is, however, just around the corner. While a need exists for further development, cost reduction, and design improvements, there are already two distinct benefits: the ability to do substantial retrofits (with 30% productivity gains) to existing aging technologies and the ability to extend the life of cells and cathodes. More importantly, however, it is a start for further development, since it opens the door for innovative multi-electrode cells.
References
1. K. Grjotheim and B.J. Welch, "Impact of Alternative Processes for Aluminium Production on Energy Requirements," Light Metals 1981, ed. G.M. Bell (Warrendale, PA: TMS, 1981), pp. 1037–1050.
2. M. Keinbourg and J.P. Cuny, "Aluminium Pechiney 180 kA Prebake Pot—From Prototype To Potline," Light Metals 1982, ed. J.E. Anderson (Warrendale, PA: TMS, 1982), pp. 449–460.
3. J. Caissy, G. Dufour, and P Lapointe, "On the Road to 325kA," Light Metals 1998, ed. B. Welch (Warrendale, PA: TMS, 1998), pp. 215–219.
4. A.S. Russell, L.L. Knapp, and W.E. Haupin, U.S. Patent 3,725,222 (April 3, 1973).
5. N. Q. Minh and N.P. Yao, "The Electrolysis of Aluminium Sulphide in Molten Fluorides," Light Metals 1984, ed. J.P. McGeer (Warrendale, PA: TMS, 1984), pp. 643–650.
6. K. Motzfeldt et al., Carbothermal Production of Aluminium (Dusseldorf, Germany: Al Verlag Pub., 1989).
7. C.E. Ransley, "The Application of the Refractory Carbides and Borides to Aluminium Reduction Cells," Extractive Met of Aluminum, Vol. 2 Aluminum (New York: Interscience, 1962), pp. 487–506.
8. C.J. McMinn, "A Review of RHM Cathode Development," Light Metals 1992, ed. E. Cutshall (Warrendale, PA: TMS, 1992), pp. 419–425.
9. H.A. Øye et al., "Properties of Colloidal Alumina-Bonded TiB2 Coating on Carbon Cathode Materials," Light Metals 1997, ed. R. Huglen (Warrendale, PA: TMS, 1997), pp. 279–286.
10. G.D. Browne et al., "TiB2 Coated Aluminium Reduction Cells: Status and Future Directions of Coated Cells in Comalco," Proc. 6th Aust. Al Smelting Tech. Workshop, ed. B.J. James, M. Skyllas-Kazacos, and B.J. Welch (Sydney, Australia: U. of N.S.W. and RACI, 1998), pp. 499–508.
11. F. Hiltmann and K. Seitz, "Titanium Diboride Plasma Coating of Carbon Cathode Materials," Light Metals 1998, ed. B. Welch (Warrendale, PA: TMS, 1998), pp. 379–390.
12. H.A. Øye et al., "Reduction of Sodium Induced Stresses in Hall Heroult Cells," Aluminium, 72 (1996).
13. B.J. Welch, "Thermochemistry of Smelting Electrolyte and Cell Operations," 17th International Course on Process Metallurgy of Aluminium (Trondheim, Norway: NTNU, May 1998), pp. 14-1–14-31.
14. T.M. Hyde and B.J. Welch, "The Gas Under Anodes in Aluminium Smelting Cells. Part I: Measuring and Modelling Bubble Resistance under Horizontally Oriented Electrodes," Light Metals 1997, ed. R. Huglen (Warrendale, PA: TMS, 1997), p. 333–340.
15. H. Kvande, "Inert Electrodes in Aluminium Electrolysis Cells," Light Metals 1999, ed. C.E. Eckert (Warrendale, PA: TMS, 1999), pp. 369–376.
16. T.R. Beck, "Production of Aluminium with Low Temperature Fluoride Melts," Light Metals 1994, ed. J.P. McGeer (Warrendale, PA: TMS, 1984), pp. 417–423.
17. T.R. Beck, I. Rouser, and J. Thonstad, "Energy Efficiency Considerations on Monopolar Versus Bipolar Fused Salt Electrolysis Cells," Light Metals 1993, ed. S.K. Das (Warrendale, PA: TMS, 1993), pp. 485–491.
18. H. Alder, U.S. Patent 3,930,967 (January 1976).
Barry J. Welch is a professor in the Chemical and Materials Engineering Department at the University of Auckland.
For more information, contact B.J. Welch, University of Auckland, Department of Chemical and Materials Engineering, Private Bag 92019, Auckland, New Zealand; telephone 64-9-373-7515; fax 64-9-393-7463; e-mail b.welch@auckland.ac.nz.
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|