COMPOSITE MATERIALS
Investigation of Thermal Residual Stresses in Layered Composite Using the Finite Element Method and X-Ray Diffraction
S. HO and E.J. LAVERNIA
A SiC particulate-reinforced 2024Al matrix composite was fabricated using a spray atomization and codeposition process to produce a layered macrostructure. The thermal macro- and microresidual stresses that develop in this layered 2024Al/SiC composite during cooling from the codeposition temperature to ambient temperature were studied using thermoelastoplastic finite element analysis. The calculated residual stresses from finite element method were compared with those obtained from X-ray diffraction (XRD), and good agreement was observed between them. The macroresidual stress distribution was very distinct for the Al and the SiC-rich layers. The macroradial stress was tensile in the Al layers and compressive in the SiC-rich layers. The macroaxial stress was found to be compressive in the vicinity of the center region of the spray-deposited material and to have mostly a continuous distribution in the Al and the SiC-rich layers. The magnitude of the macroaxial stress was noted to decrease with increasing deposition thickness from the bottom. In addition, the spray-deposited material exhibited the highest macro-von Mises' stress around the outer edge of the deposited material. The microradial stress was in a compressive state in the SiC particulate and in the Al matrix in the vicinity of the SiC particulate. The SiC particulate exhibited compressive microhoop stress, whereas the Al matrix exhibited tensile microhoop stress. The micro-von Mises' stress was of the highest value at the interface between the SiC particulate and the Al matrix.
HYDROMETALLURGY
The Leaching Kinetics of Chalcopyrite (CuFeS2) in Ammonium Iodide Solutions with Iodine
Y. CHARLES GUAN and KENNETH N. HAN
The leaching kinetics of chalcopyrite (CuFeS2) in ammonium iodide solutions with iodine has been studied using the rotating disc method. The variables studied include the concentrations of lixiviants, rotation speed, pH of the solution, reaction temperature, and reaction product layer. The leaching rate was found to be independent of the disc rotating speed. The apparent activation energy was measured to be about 50 kJ/mole from 16°C to 35°C, and 30.3 kJ/mole from 35°C to 60°C. The experimental findings were described by an electrochemical reaction-controlled kinetic model: rate = k [NH3]0.69[OH-]0.42[I-3]0.5.
Preparation of Ultra-High-Purity Copper by Anion Exchange
TAMAS KEKESI, KOUJI MIMURA, YUKIO ISHIKAWA, and MINORU ISSHIKI
Equilibrium studies of anion-exchange distribution pointed out the feasibility of eliminating virtually all the impurities from a copper chloride solution containing copper initially in the monovalent state. Elution tests proved that the proposed anion-exchange separation can offer extremely high degrees of purification by utilizing both the mono- and divalent states of copper and by properly controlling the HCl concentration during the procedure. Initially, the Cu(I) chlorocomplexes are fixed and most of the impurities are eliminated, followed by an in situ oxidation for eluting copper in the Cu(II) state while retaining the rest of the elements in the column. Copper was extracted from the purified and oxidized CuCl2-HCl solution by evaporation to dryness and hydrogen reduction. The produced copper sponge was melted in Ar-H2 plasma, followed by cold rolling, cutting, and wire drawing to obtain samples for glow discharge mass spectrometry (GDMS) analysis.
The Leaching of Nickeliferous Laterite with Ferric Chloride
NORMAN D.H. MUNROE
Several experiments were conducted to investigate the extraction of nickel from nickeliferous laterite by ferric chloride solutions as a function of pulp density, solution composition, and temperature. Solubility relationships for goethite and nickel laterite in aqueous solution were reviewed in terms of leaching rates and reaction mechanisms. Generally, the amount of nickel extracted increased with temperature, the amount of "free acid," and ferric chloride concentration; however, the amount was inhibited by ferrous chloride. In this investigation, as much as 96 pct of the available nickel was extracted by ferric chloride solution. Nickel extraction was found to be more dependent on ferric chloride concentration than on the concentration of hydrochloric acid. Mechanistically, nickel extraction occurred by the formation of an intermediate ferric chloride complex, which was then hydrolyzed to hematite.
PYROMETALLURGY
Dissolution of Hard-Alpha Inclusions in Liquid Titanium Alloys
J.P. BELLOT, B. FOSTER, S. HANS, E. HESS, D. ABLITZER and A. MITCHELL
Canadian and French university teams have joined efforts in carrying out an experimental and theoretical study of the dissolution behavior of the hard-alpha inclusion in liquid titanium alloys. Synthetic hard-alpha dense particles of up to 6 wt pct nitrogen and nitrided sponge of up to 15 wt pct nitrogen were partially dissolved in a titanium or a titanium alloy bath. The metallographic examinations and microprobe analysis show that the dissolution process is always controlled by the outward diffusion of nitrogen into the bath through an external layer of beta phase. The growth of this beta phase layer depends on the velocity of liquid flow in the bath and can lead to an initial increase in the inclusion size. For porous particles, the diffusion of nitrogen from the pellet matrix to the infiltrations gradually leads to a partial densification of the inclusion. A numerical representation of the dissolution problem was developed, including the transient diffusion of nitrogen through intermediate solid phases. The comparison is good between the numerical simulations, the experimental measurements, and the dissolution kinetics given in the literature.
Thermodynamic Optimization of the Systems PbO-SiO2, PbO-ZnO, ZnO-SiO2 and PbO-ZnO-SiO2
EVGUENI JAK, SERGEI DEGTEROV, PING WU, PETER C. HAYES, and ARTHUR D. PELTON
Liquidus-phase equilibrium data of the present authors for the PbO-ZnO-SiO2 system, combined with phase equilibrium and thermodynamic data from the literature, were optimized to obtain a self-consistent set of parameters of thermodynamic models for all phases. The modified quasichemical model was used for the liquid slag phase. From these model parameters, the optimized ternary-phase diagram was back-calculated.
Reaction Mechanism on the Smelting Reduction of Iron Ore by Solid Carbon
JAE-CHEOL LEE, DONG-JOON MIN, and SUNG-SOO KIM
The kinetics of the smelting reduction of iron ore by a graphite crucible and carbon-saturated molten iron was investigated between 1400°C and 1550°C, and its reaction phenomena were continuously observed in situ by X-ray fluoroscopy. In the smelting reduction by graphite, it was shown from the observation results that the smelting reduction reaction proceeded by the following two stages: an initial quiet reduction without foaming (stage I) and a following highly active reduction with severe foaming (stage II). At 1500°C, by the graphite crucible, the reduction rate of iron ore was found to be 8.88 X 10-5 mol/cm2·s, and by the molten iron, 8.25 X 10-5 mol/cm2·s. The activation energies for the reduction by the graphite crucible and the molten iron were 24.1 and 22.9 kcal/mol, respectively. Based on the results of kinetic research and X-ray fluoroscopic observations, it can be concluded that these two types of smelting reduction reactions of iron ore by the graphite crucible and by the molten iron are essentially the same.
Copper Removal from Carbon-Saturated Molten Iron with Al2S3-FeS Flux
RYOKICHI SHIMPO, YUICHI FUKAYA, TAKEHIRO ISHIKAWA, and OSAMU OGAWA
Copper removal from carbon-saturated molten iron to Al2S3-FeS flux was experimentally investigated in the temperature range from 1473 to 1573 K. The maximum copper distribution ratio between the Al2S3-FeS flux and the ferrous alloy was about 28 at the composition where the molar ratio of Al to Fe in the flux was around 2. The distribution ratio was no less than 25 as long as the copper content of the flux was less than 10 mass pct. The sulfur content in the ferrous alloy in equilibrium with the Al2S3-FeS flux was higher than that obtained by using Na2S-FeS flux, and it was concluded that the high copper distribution ratio of the Al2S3-FeS flux was brought about by the high activity coefficient of FeS in the flux. In experiments for recovering copper from the flux, copper in Al2S3-FeS-Cu2S flux was reduced by metallic aluminum at 1473 K. The FeS in the flux was primarily reduced and, after that, the copper was recovered in the form of Cu-Al-Fe alloy. The residual copper content in the flux could be decreased to less than 1 mass pct when the aluminum content in the alloy was higher than 40 mass pct. A process for copper removal from molten iron is proposed, which uses successive contacts between the Al2S3-FeS flux and molten iron at several stages with counterflow operation. It is suggested that 1 mass pct Cu in molten iron can be reduced to approximately 0.1 mass pct Cu using 100 kg flux/ton iron; the amount of aluminum required for the iterative use of the flux is about 10 kg/ton iron. By the recycling use of this Al2S3-FeS flux, it is suggested that copper removal from molten iron using the sulfide flux can be more effective.
The Kinetics of Selenium Removal from Molten Copper by Powder Injection
Y.F. ZHAO and G.A. IRONS
Chemical thermodynamic calculations show that selenium removal from copper melts using sodium carbonate (soda ash) is only effective under reducing conditions. Reducing conditions can be generated by carbon, but even more effectively by calcium carbide which has not been used previously for such a purpose. To clarify the kinetics of these multiphase, multicomponent reactions, various mixtures were either placed on top of or injected into 70 kg heats of molten copper. The following reagents were found to be effective in removing selenium: soda ash-graphite mixtures, calcium carbide, and calcium carbide-soda ash mixtures, in increasing order of effectiveness. Experiments were also performed with synthetic blister copper containing oxygen, selenium, tellurium, bismuth, nickel, silver, and lead. As expected from the thermodynamic analysis, only the first three of these elements were removed. A mathematical model was developed to describe the diffusion-controlled reaction kinetics of selenium and oxygen removal at calcium carbide particle interfaces. Very good agreement between the model and experiments was achieved for the reaction paths of selenium and oxygen when 35 pct of the particles were in contact with the melt. The utilization of powder varied over a wide range (0 to 10 pct), depending on the selenium and oxygen contents. The industrial implications of this work are discussed in terms of multielement removal, refractory erosion, temperature loss, and reagent utilization.
TRANSPORT PHENOMENA
Bubble and Liquid Flow Characteristics in a Wood's Metal Bath Stirred by Bottom Helium Gas Injection
MANABU IGUCHI, HIROHIKO TOKUNAGA, and HIDEO TATEMICHI
A model study was carried out to elucidate bubble and liquid flow characteristics in the reactor of metals refining processes stirred by gas injection. Wood's metal with a melting temperature of 70°C was used as the model of molten metal. Helium gas was injected into the bath through a centered single-hole bottom nozzle to form a vertical bubbling jet along the centerline of the bath. The bubble characteristics specified by gas holdup, bubble frequency, and so on were measured using a two-needle electroresistivity probe, and the liquid flow characteristics, such as the axial and radial mean velocity components, were measured with a magnet probe. In the axial region far from the nozzle exit, where the disintegration of rising bubbles takes place and the radial distribution of gas holdup follows a Gaussian distribution, the axial mean velocity and turbulence components of liquid flow in the vertical direction are predicted approximately by empirical correlations derived originally for a water-air system, although the physical properties of the two systems are significantly different from each other. Under these same conditions, those turbulent parameters in high-temperature metals refining processes should thus be accurately predicted by the same empirical correlations.
PHYSICAL CHEMISTRY
The Intrinsic Thermal Decomposition Kinetics of SrCO3 by a Nonisothermal Technique
I. ARVANITIDIS, DU SICHEN, H.Y. SOHN, and S. SEETHARAMAN
The kinetics of the decomposition of SrCO3 in argon to SrO and CO2 were studied in the temperature range 1000 to 1350 K. The thermal decomposition was followed simultaneously by thermogravimetric analysis (TGA) and differential thermal analysis (DTA) during linear heating. By using a nonisothermal method, the complete rate expression was determined from a relatively small number of experimental runs. Shallow beds of fine synthetic powder as well as thin flakes of pressed powder were employed to obtain the kinetics of decomposition in the absence of heat- and mass-transfer effects. The thermal decomposition started at about 1000 K. The recommended rate expression for the SrCO3 decomposition is
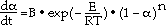
where  is the ratio between the actual weight change and the theoretical final weight change, d
is the ratio between the actual weight change and the theoretical final weight change, d /dt is the time derivative of
/dt is the time derivative of  , B is a rate constant in s-1, E is the activation energy in J·mol-1, R is the gas constant in J·K-11·mol-1, T is the temperature in kelvin, and n is a factor depending on the geometry of the particles. The activation energy, E, for the decomposition of SrCO3 was evaluated to be 210 kJ/mol. Curves of calculated
, B is a rate constant in s-1, E is the activation energy in J·mol-1, R is the gas constant in J·K-11·mol-1, T is the temperature in kelvin, and n is a factor depending on the geometry of the particles. The activation energy, E, for the decomposition of SrCO3 was evaluated to be 210 kJ/mol. Curves of calculated  vs temperature agree well with the experimental results.
vs temperature agree well with the experimental results.
Reduction of Titanium Dioxide in a Nonequilibrium Hydrogen Plasma
DANIEL E. BULLARD and DAVID C. LYNCH
Plasma processing offers improved thermodynamics and kinetics over conventional, thermal processing. In the current work, the reduction of TiO2 was investigated in a moderate-pressure (p < 46 torr) nonequilibrium hydrogen plasma at temperatures below 1273 K; the effect of plasma power, plasma pressure, time, and applied voltage on the extent of the reduction was examined. Reduction of powdered TiO2 at the surface of a packed bed has produced up to 60 pct conversion of TiO2 to Ti2O3 in only 5 minutes of plasma-specimen contact. While the plasma-assisted reduction occurs at the surface, the reduction of TiO2 to Ti50O99 within the interior of the bed by diatomic hydrogen establishes a value of PH2O that leads to the reoxidation of the Ti2O3. The continued reduction of the surface material by monatomic hydrogen from the plasma balances this oxidation process, and a steady-state condition is established. When the interior of the bed is completely reduced to Ti50O99, the partial pressure of water vapor declines, and further reduction of Ti2O3 at the surface can proceed. It is hypothesized that the reduction process involves the formation of a Magneli-like oxide between Ti2O3 and TiO.
Detailed Assessment of Integral Thermodynamic Quantities of Liquid Bi-Sn Alloy Solution System
SEUNG-AM CHO and JOSE LUIS OCHOA
We calculated in great detail all the relative partial molar thermodynamic quantities of Bi,

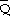 xsBi, and
xsBi, and

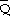 Bi, where
Q =
H, S, and G, of the molten Bi-Sn system from their experimental counterparts,
Bi, where
Q =
H, S, and G, of the molten Bi-Sn system from their experimental counterparts,

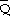 xsSn and
xsSn and

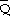 Sn,
using the authentic Chiotti integration and assessed their unequivocal limiting values at infinite dilution of both components,
Sn,
using the authentic Chiotti integration and assessed their unequivocal limiting values at infinite dilution of both components,

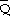 xsi(°) and
xsi(°) and

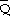 °,
by the genuine Chiotti derivative. Thermodynamic consistency tests were satisfied. While the excess partial quantities,
°,
by the genuine Chiotti derivative. Thermodynamic consistency tests were satisfied. While the excess partial quantities,

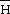 i
i

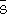 xsi, and
xsi, and

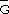 xsi, oscillate with composition, their excess integral quantities,
xsi, oscillate with composition, their excess integral quantities,
 Hm
Hm
 Sxsm, and
Sxsm, and
 Gxsm, are rather bland; and the integral quantities,
Gxsm, are rather bland; and the integral quantities,
 Sm, and
Sm, and
 Gm, are nearly symmetrical with composition.
The Bi-Sn solution is definitel nonregular and the solution reaction is exothermic
(
Gm, are nearly symmetrical with composition.
The Bi-Sn solution is definitel nonregular and the solution reaction is exothermic
( Hm<0). Atomistically, the solution has a weak segregation tendency, shown by the values in the relation T|
Hm<0). Atomistically, the solution has a weak segregation tendency, shown by the values in the relation T|
 Sxsm| >
Sxsm| >
 Gxsm > |
Gxsm > |
 Hm|.
Although the excess integral molar heat capacity of our solution is zero,
Hm|.
Although the excess integral molar heat capacity of our solution is zero,
 Cxsp,m = 0, the solution does not belong to the Guggenheim's athermal type by definition.
Cxsp,m = 0, the solution does not belong to the Guggenheim's athermal type by definition.
Activities in the System Cryolite-Alumina
ERNEST W. DEWING and JOMAR THONSTAD
Reasons are outlined for thinking that accepted values for the activity of Al2O3 in solution in cryolite are in error, and a new analysis is made of literature cryoscopic data. A general treatment is given for the effect of incongruent melting caused by the nonstoichiometry of solid cryolite. The effect is substantial, and accurate calculations require a knowledge of the effect of the solute on the activity of NaF in the solution, since that controls the composition of the solid separating. The results in the case of alumina as solute show that the slope of the log (activity) vs log (concentration) plot is about 3 in dilute solution and 1.5 in concentrated solution, corresponding to species containing 1 and 2 atoms of oxygen, respectively. Any calculations employing the earlier relation  Al2O3
Al2O3  (wt pct Al2O3)2.77, which was based on electrochemical measurements of activity, need to be redone.
(wt pct Al2O3)2.77, which was based on electrochemical measurements of activity, need to be redone.
Thermodynamics of the System NaF-AlF3: Part VII. Non-Stoichiometric Solid Cryolite
ERNEST W. DEWING
Earlier data are recalculated to give the boundaries of the homogeneous field of solid cryolite. The equilibrium constant
K = x3 · (aNaF)2/[18(1 - x/3)2 · (1 - x/2)]
where the composition of the material is (3 - x)NaF · AlF3, is
log10 (K) = -12,543/T + 5.109
The activity of NaF, aNaF, is referred to the solid standard state. The standard Gibbs energy of fusion of stoichiometric cryolite is
 G°/J = 119,331 - 94.695 T
G°/J = 119,331 - 94.695 T
The Solubility of ZnO and ZnAl2O4 in Cryolite Melts
E.W. DEWING, S. ROLSETH, L. STØEN, and J. THONSTAD
The solubilities of ZnO and ZnAl2O4 in cryolite-alumina melts at 1020°C have been measured. ZnAl2O4 is the stable solid phase at Al2O3 contents greater than 1.45 ± 0.2 wt pct. The results can be represented (in terms of wt pct Zn in solution and in the range 1.2 to 13.6 pct Al2O3) by
log (pct Zn) = 0.491 + 1.90 log (pct Al2O3)
where (pct Al2O3) includes the alumina formed by the dissolution reaction. The results are discussed in terms of the variation of the activity of alumina with its concentration and are consistent with the dissolution mechanism:
3ZnO + 2AlF3 = 3ZnF2 + Al2O3
The activity coefficient of ZnF2 in dilute solution with respect to a pure liquid standard state is 0.14.
Thermodynamics of Mixed Oxide Compounds, Li2O · Ln2O3 (Ln = Nd or Ce)
K.V. GOURISHANKAR, G.K. JOHNSON, and IRVING JOHNSON
The thermodynamics of mixed oxide compounds of the type Li2O · Ln2O3 (Ln = Nd or Ce) has been studied by measuring the lanthanide content of molten LiCl-Li2O-Li solutions in equilibrium with the solid mixed oxide compound at 923 K. The observed total lanthanide mole fraction in the molten salt when in equilibrium with both solid Ln2O3 and LiLnO2 was found to be 1.0 X 10-4 and 5.9 X 10-4 for Ln = Nd and Ce, respectively. The calculated mole fractions of LiLnO2 in the molten salt when the solid mixed oxide compound was present are 2.6 X 10-5 and 2.1 X 10-4 for LiNdO2 and LiCeO2, respectively. The unexpectedly large "solubilities" of the sesquioxides in the presence of Li2O can be explained by the formation of complex oxide species, LiLnO2 and Li3LnO3, in the molten salt solutions. The equilibrium constants and the free energies of formation for these species were derived from the measured data. The free energies of formation of the solid mixed oxide compounds at 923 K were found to be -1014 ± 1.5 kJ/mol for LiNdO2 and -1006 ± 11.0 kJ/mol for LiCeO2.
Thermodynamics of MnO, FeO, and Phosphorus in Steelmaking Slags with High MnO Contents
A.T. MORALES and R.J. FRUEHAN
The thermodynamics of manganese oxide and iron oxide and the phosphate capacity of CaO-SiO2-MnO-Fe O-P2O5-MgOsat slags with high MnO contents relevant to the smelting of MnO ores in steelmaking were investigated. Previous data were limited to about 5 pct MnO, whereas in this study MnO contents up to 25 pct were studied. The activity of MnO showed positive deviation from ideal behavior and increased with basicity, while that of Fe
O-P2O5-MgOsat slags with high MnO contents relevant to the smelting of MnO ores in steelmaking were investigated. Previous data were limited to about 5 pct MnO, whereas in this study MnO contents up to 25 pct were studied. The activity of MnO showed positive deviation from ideal behavior and increased with basicity, while that of Fe O decreased with basicity. This is reflected in that the manganese distribution ratio at a given Fe
O decreased with basicity. This is reflected in that the manganese distribution ratio at a given Fe O content decreases with basicity and high Mn in the metal is favored by high basicity. CaF2 additions up to 4 pct did not affect the activities of MnO and Fe
O content decreases with basicity and high Mn in the metal is favored by high basicity. CaF2 additions up to 4 pct did not affect the activities of MnO and Fe O. The activity coefficient of P2O5 decreases and the phosphate capacity increases with basicity. There was not adverse effect of high MnO contents on the dephosphorizing abilities of the slags.
O. The activity coefficient of P2O5 decreases and the phosphate capacity increases with basicity. There was not adverse effect of high MnO contents on the dephosphorizing abilities of the slags.
Enthalpies of Formation of Liquid Binary (Copper + Iron, Cobalt, and Nickel) Alloys
I.V. NIKOLAENKO and M.A. TURCHANIN
The enthalpies of formation of liquid binary (Cu + Fe, Co, Ni) alloys are studied by direct reaction calorimetry in the whole range of compositions at 1873, 1823, and 1753 K, respectively. The integral molar enthalpies of mixing are found to be positive in all three systems with the maximum values approaching 10.8 ± 0.7 kJ/mol-1 at xFe = 0.43, 7.1 ± 0.9 kJ/mol-1 at xCo = 0.55, and 3.7 ± 0.5 kJ/mol-1 at xNi = 0.53. Partial molar enthalpies at infinite dilution constitute 59.4 ± 3.3 kJ/mol-1 for iron, 44.3 ± 4.1 kJ/mol-1 for cobalt, and 14.9 ± 2.2 kJ/mol-1 for nickel in liquid copper. Similar values for copper in liquid iron, cobalt, and nickel are 36.6 ± 3.9, 45.3 ± 6.0, and 17.7 ± 4.4 kJ/mol-1, respectively. The results are compared with the thermodynamic data available in literature and discussed in connection to the equilibrium-phase diagrams. In particular, decreasing from Cu-Fe to Cu-Ni liquid alloys positive values of the excess thermodynamic functions of mixing are fully in accord with the growing stability of phases in these systems. The excess entropies of mixing are estimated by combining the established enthalpies with carefully selected literature data for the excess Gibbs functions. Analysis of possible contributions to the enthalpies of mixing indicates that the experimentally established regularity in  H values along the 3d series is likely to arise from the difference in d-band width and d-electron binding energy of the alloy constituents.
H values along the 3d series is likely to arise from the difference in d-band width and d-electron binding energy of the alloy constituents.
Deoxidation Equilibria of Calcium and Magnesium in Liquid Iron
HIROKI OHTA and HIDEAKI SUITO
Calcium-oxygen and magnesium-oxygen equilibria in liquid iron saturated with CaO-SiO2-Al2O3-MgO slags were studied at 1873 K using CaO, Al2O3, and MgO crucibles. The applicability of the first-order and second-order interaction coefficients between Ca and O and between Mg and O was studied by comparing the Ca-O and Mg-O equilibria observed in the present and previous experiments with the calculated ones from the respective interaction coefficients. As a result, the interaction coefficients obtained in the present work by using a new method were found to explain the measured solubilities of CaO and MgO. The phase stability region in Fe-Al M (M = Ca, Mg)-O system was described at 1873 K.
A High-Accuracy, Calibration-Free Technique for Measuring the Electrical Conductivity of Molten Oxides
SUSAN L. SCHIEFELBEIN and DONALD R. SADOWAY
A high-accuracy, calibration-free technique to measure the electrical conductivity of molten oxides has been developed--the coaxial cylinders technique. Because the melt under investigation comes in contact only with metal and not with anything dielectric, the new technique enables the measurement of the electrical properties of liquids inaccessible by classical high-accuracy techniques. Two coaxial cylindrical electrodes are immersed in the melt to an arbitrary initial depth, and ac impedance is measured over a wide range of frequency. The electrodes are then immersed deeper, and ac impedance is again measured over the same range of frequency. This process is repeated at multiple immersions. The electrical conductivity is calculated from the change in measured conductance with immersion. The temperature dependence of electrical conductivity has been measured for two oxide melts: (M) 50.95 pct CaO, 12.51 pct MgO, 36.54 pct SiO2, 1733 K < T < 1843 K; and (S) 24.59 pct CaO, 26.15 pct MgO, 49.26 pct SiO2, 1763 K < T < 1903 K, where concentration is expressed in mole percent. Both melts exhibited behavior characteristic of ionic liquids.
Thermodynamics of Phosphorus in Molten Si-Fe and Si-Mn Alloys
SHIGERU UEDA, KAZUKI MORITA, and NOBUO SANO
The thermodynamics of phosphorus in molten Si-Fe and Si-Mn alloys has been investigated at 1723 K by equilibrating the alloys in a controlled phosphorus partial pressure. The activity coefficient of phosphorus in each alloy shows a maximum value at a certain composition due to a strong interaction between silicon and iron and between silicon and manganese. Interaction coefficients between phosphorus and iron in molten silicon were found to be
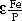 = 7.43 and
= 7.43 and
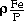 = -16.4 (0
= -16.4 (0
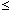 XFe
XFe
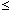 0.65), and those between phosphorus and manganese were
0.65), and those between phosphorus and manganese were
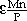 = 12.0 and
= 12.0 and
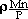 = -22.2 (0
= -22.2 (0
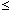 XMn
XMn
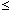 0.5). Further discussion has revealed that the Si-Fe-P and Si-Mn-P systems approximately conform to a regular solution within the composition ranges investigated in the present work.
0.5). Further discussion has revealed that the Si-Fe-P and Si-Mn-P systems approximately conform to a regular solution within the composition ranges investigated in the present work.
Determination of Kinetic Parameters Using Differential Thermal Analysis--Application to the Decomposition of CaCO3
X. XIAO, DU SICHEN, H.Y. SOHN, and S. SEETHARAMAN
An analytical method has been developed to determine the kinetic parameters of a chemical reaction involving a substantial enthalpy change using differential thermal analysis (DTA). The theoretical treatment is based on fundamental equations considering the heat balance. The analytical derivation was simplified by carefully choosing the experimental conditions. The method was applied to the decomposition of CaCO3 in argon gas. The activation energy of the decomposition of CaCO3 evaluated using the present approach is in very good agreement with the result obtained from the thermogravimetric analyses (TGA) carried out simultaneously with the DTA measurements. The limitation of the technique includes maintaining the temperature rise of the sample small enough not to significantly affect temperature reading but large enough to ensure accurate measurement of the heat generation.
Communication: Solutions of CeO2 in Cryolite Melts
ERNEST W. DEWING and JOMAR THONSTAD
Communication: Dependence of Carbon Solubility on Oxygen Partial Pressure for 80 Mass Pct BaO-MnO and CaOsatd-B2O3 Slags
MAMIKO MORI, KAZUKI MORITA, and NOBUO SANO
SOLIDIFICATION
Modeling of Solidification of Metal-Matrix Particulate Composites with Convection
R.J. FELLER and C. BECKERMANN
A multiphase model for the alloy solidification of metal-matrix particulate composites with convection is developed. Macroscopic transport equations are written for each phase, with unknown parameters modeled through supplementary relations pertinent to the solidification of a binary alloy matrix containing a stationary solid phase and generally nonstationary spherical particles. The model is applied to various one- and two-dimensional systems containing an Al-7 wt pct Si/SiC composite. One-dimensional sedimentation results in nonclustering and clustering particle systems show good agreement with experiments. One-dimensional composite solidification results illustrate the effect of particle clustering and cooling direction on the final macroscopic particle distribution. Two-dimensional results in various unreinforced and reinforced systems illustrate macroscopic particle segregation and its effect on buoyancy-driven melt convection and species macrosegregation. Results indicate a nearly uniform particle distribution for relatively small particles due to negligible particle settling prior to entrapment. For relatively large particles, significant particle settling prior to entrapment results in large denuded and packed zones in the casting. Fluid flow and macrosegregation during solidification are substantially reduced in the presence of particles, due to the relatively large interfacial drag exerted on the liquid by the stationary mush and particle phases.
Models for the Isothermal Coarsening of Secondary Dendrite Arms in Multicomponent Alloys
QINGYOU HAN, HANQI HU, and XUEYOU ZHONG
Models for the isothermal coarsening of secondary dendrite arms for binary alloys are simply expanded to correlate the secondary dendrite arm spacing to local solidification time, melting temperature of the liquid, and the properties of the solute elements in multicomponent alloys. An equation is derived. Calculations using the equation show reasonable qualitative agreement with experiment.
SOLID STATE REACTIONS
Influence of Short Circuiting on the Kinetics and Mechanism of Iodide Film Growth on Ag and Cd-Doped Ag
S.C. KUIRY, S.K. ROY, and S.K. BOSE
Results of iodination studies on pure Ag and Ag with 6000 ppm of Cd under normal and short-circuit conditions in the temperature and iodine pressure ranges of 333 to 373 K and 0.067 to 6.078 kPa, respectively, are reported. Under all experimental conditions, the iodide film growth kinetics conform to parabolic rate law. The iodide films have been characterized by scanning electron microscopy (SEM), electron probe microanalysis (EPM), X-ray diffraction (XRD), and Auger electron spectroscopy (AES) analyses. The effect of a higher valent dopant like Cd in Ag is observed to decrease the rate of normal iodination, which suggests that the film growth process is controlled by migration of electron holes across the iodide layers. The presence of a short-circuit path enhances the rate of iodination for pure Ag. However, the iodination rate of Cd-doped Ag under short-circuit mode is found to be further enhanced compared to that for pure Ag. This has been explained on the basis of the ion migration mechanism. The pressure dependence of rate constants is found to follow a relation like kP

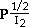 for pure Ag and Cd-doped Ag under normal conditions. The corresponding relations under short-circuit mode are observed to be kP
for pure Ag and Cd-doped Ag under normal conditions. The corresponding relations under short-circuit mode are observed to be kP

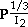 and kP
and kP

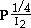 for Ag and Cd-doped Ag, respectively. Arrhenius plots have yielded activation energy values of 25.8 and 15.2 kJ·mol-1 under normal and short-circuit conditions, respectively. The kinetic results of parabolic film growth have been explained with the help of defect equilibria, considering the predominance of Frenkel defects in pure and doped AgI lattices. The mechanism of film growth processes has been confirmed to be Wagner's electrochemical potential gradient-induced migration of defect species across the iodide layer.
for Ag and Cd-doped Ag, respectively. Arrhenius plots have yielded activation energy values of 25.8 and 15.2 kJ·mol-1 under normal and short-circuit conditions, respectively. The kinetic results of parabolic film growth have been explained with the help of defect equilibria, considering the predominance of Frenkel defects in pure and doped AgI lattices. The mechanism of film growth processes has been confirmed to be Wagner's electrochemical potential gradient-induced migration of defect species across the iodide layer.
MATERIALS PROCESSING
A Flame Process for Synthesis of Unagglomerated, Low-Oxygen Nanoparticles: Application to Ti and TiB2
R.L. AXELBAUM, D.P. DUFAUX, C.A. FREY, and S.M.L. SASTRY
A gas-phase flame process for synthesizing unagglomerated nanoparticles of metals, intermetallics, ceramics, and composites is described. Employing this process, titanium and titanium boride have been synthesized by the vapor-phase reaction of sodium with titanium tetrachloride and a 1:2 mixture of titanium tetrachloride and boron trichloride, respectively. To minimize agglomeration and protect the particles from postflame oxidation, the NaCl by-product is allowed to condense onto the particles in situ, yielding NaCl-encapsulated particles. In this way, stable, unagglomerated Ti and TiB2 nanoparticles have been produced and the encapsulated powders have been handled in air without oxidation. Particle size has also been varied with the encapsulation process, and titanium particles with mean sizes of 10 and 60 nm have been produced by varying operating conditions. The NaCl has been removed by water washing as well as vacuum annealing. Thermodynamic results show that the sodium/halide process is applicable for synthesis of many materials, with yields approaching 100 pct under a wide range of operating conditions. Similarly, the encapsulation process is generally applicable, making the sodium/halide flame and encapsulation process a viable one for large-scale synthesis of environmentally insensitive nanopowders.
A Thermal Elastic-Plastic Finite-Element Analysis to Roll-Life Prediction on the Twin Roll Strip Continuous Casting Process
C.G. KANG and Y.D. KIM
The rolling force and roll deformation behavior in the twin-roll-type strip continuous casting process have been computed to estimate the thermal characteristics of a caster roll. To calculate the rolling force, the relationship between the flow stress and strain for a roll material and a casting alloy are assumed as a function of the strain rate and temperature, because the mechanical properties of casting materials depend on temperature. The three-dimensional (3-D) thermal elastic-plastic analysis of a cooling roll has also been carried out, to obtain roll stress and plastic strain distributions, with the commercial finite-element analysis package of ANSYS. Temperature field data for a caster roll, provided by the authors, were used to estimate the roll deformation. Therefore, numerical models considering the thermal and rolling forces have been developed to estimate the roll life. Roll life considering the thermal cycle is calculated using thermal elastic-plastic analysis results. The roll life is proposed in terms of roll revolution in the caster roll models with and without the fine crack failure on the roll surface. To obtain plastic strain distributions of the caster roll, thermomechanical properties of a roll sleeve with a copper alloy are obtained by a uniaxial tensile test for variation of temperature. The proposed analysis techniques have improved in caster roll design.
Analysis of Multicomponent Evaporation in Electron Beam Melting and Refining of Titanium Alloys
A. POWELL, J. VAN DEN AVYLE, B. DAMKROGER, J. SZEKELY, and U. PAL
Experimental evidence and a mathematical model are presented to evaluate the effect of beam-scan frequency on composition change in electron-beam melting of titanium alloys. Experiments characterized the evaporation rate of commercially pure (CP) titanium and vapor composition over titanium alloy with up to 6 wt pct aluminum and 4.5 wt pct vanadium, as a function of beam power, scan frequency, and background pressure. These data and thermal mapping of the hearth melt surface are used to estimate activity coefficients of aluminum and vanadium in the hearth. The model describes transient heat transfer in the surface of the melt and provides a means of estimating enhancement of pure titanium evaporation and change in final aluminum composition due to local heating at moderate beam-scan frequencies.
Effect of Pd, Cu, and Ni Additions on the Kinetics of NiCl2 Reduction by Hydrogen
S.R. STOPIC, I.B. ILIC, and D.P. USKOKOVIC
Differential thermal analysis (DTA) and thermal gravimetric analysis (TGA), at a heating rate of 10°C/min, revealed a complete reduction of NiCl2 by hydrogen in a temperature interval of 375°C to 450°C. However, addition of 0.1 mass pct of Pd, Cu, or Ni to the sample caused the reduction to occur at considerably lower temperatures, in the rather narrow range of 315°C to 370°C. The activation energy of NiCl2 reduction by hydrogen (between 300°C and 550°C) without additives is 54 kJ/mol, and with Pd and Cu or Ni added, under isothermal conditions (from 260°C to 380°C), is 33 and 50 kJ/mol, respectively. These values confirm a positive effect of additives on the reduction kinetics. The positive effect of Pd is a consequence of the dissociation and spillover of hydrogen, whereas in the case of Cu and Ni(HCOO)2, it is manifested in a decrease in bonds energy in the nickel lattice because of good Cu solubility, and in the formation of artificial nickel nuclei that intensify the reduction, respectively. Scanning electron microscopy (SEM) analysis of nickel powders obtained under isothermal conditions shows relatively rounded spherical particles (0.321 to 0.780 µm in size) of powder samples with additives, and particles of irregular shape (2.085 µm mean size) of the sample without additives. This illustrates the positive effect of Pd, Cu, or Ni added in the reduction process, in decreasing the size of nickel particles and in the production of a more uniform particle shape.
MATHEMATICAL MODELING
Modeling of Solidification of Molten Metal Droplet during Atomization
Y.H. SU and C.-Y.A. TSAO
A mathematical model to describe the solidification behavior of an atomized droplet during flight, in terms of nucleation temperature, recalescence temperature, nucleation position, solid fraction at nucleation temperature, and droplet temperature and velocity, is formulated. The concept of transient nucleation is applied to model the short nucleation event. A maximum droplet velocity exists, beyond which the droplet velocity shows an inflection phenomenon during the flight. The velocity of smaller droplets is higher at a shorter flight distance and lower at a longer flight distance. Variations of the gas flow patterns have more effects on smaller droplets, and the effects are more significant at a longer flight distance. A minimum surface heat-transfer coefficient exists as the droplet flies. Prior to nucleation or recalescence, smaller droplets have lower temperature at a given flight distance. Smaller droplets have lower nucleation temperature. Medium-size (around 80-;jmm) droplets fly over the shortest flight distance before the nucleation starts. Smaller droplets have a larger solid fraction at the end of recalescence. Atomization gas has more effects on the droplet momentum than on the heat content of the droplet.
Direct questions about this or any other Metallurgical and Materials Transactions page to mettrans@andrew.cmu.edu.

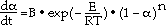
 is the ratio between the actual weight change and the theoretical final weight change, d
is the ratio between the actual weight change and the theoretical final weight change, d
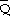 xsBi, and
xsBi, and
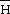 i
i
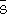 xsi, and
xsi, and
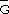 xsi, oscillate with composition, their excess integral quantities,
xsi, oscillate with composition, their excess integral quantities,
 (wt pct Al2O3)2.77, which was based on electrochemical measurements of activity, need to be redone.
(wt pct Al2O3)2.77, which was based on electrochemical measurements of activity, need to be redone.
 O-P2O5-MgOsat slags with high MnO contents relevant to the smelting of MnO ores in steelmaking were investigated. Previous data were limited to about 5 pct MnO, whereas in this study MnO contents up to 25 pct were studied. The activity of MnO showed positive deviation from ideal behavior and increased with basicity, while that of Fe
O-P2O5-MgOsat slags with high MnO contents relevant to the smelting of MnO ores in steelmaking were investigated. Previous data were limited to about 5 pct MnO, whereas in this study MnO contents up to 25 pct were studied. The activity of MnO showed positive deviation from ideal behavior and increased with basicity, while that of Fe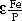 = 7.43 and
= 7.43 and
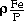 = -16.4 (0
= -16.4 (0
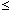 XFe
XFe
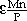 = 12.0 and
= 12.0 and
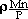 = -22.2 (0
= -22.2 (0
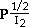 for pure Ag and Cd-doped Ag under normal conditions. The corresponding relations under short-circuit mode are observed to be kP
for pure Ag and Cd-doped Ag under normal conditions. The corresponding relations under short-circuit mode are observed to be kP
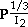 and kP
and kP
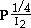 for Ag and Cd-doped Ag, respectively. Arrhenius plots have yielded activation energy values of 25.8 and 15.2 kJ·mol-1 under normal and short-circuit conditions, respectively. The kinetic results of parabolic film growth have been explained with the help of defect equilibria, considering the predominance of Frenkel defects in pure and doped AgI lattices. The mechanism of film growth processes has been confirmed to be Wagner's electrochemical potential gradient-induced migration of defect species across the iodide layer.
for Ag and Cd-doped Ag, respectively. Arrhenius plots have yielded activation energy values of 25.8 and 15.2 kJ·mol-1 under normal and short-circuit conditions, respectively. The kinetic results of parabolic film growth have been explained with the help of defect equilibria, considering the predominance of Frenkel defects in pure and doped AgI lattices. The mechanism of film growth processes has been confirmed to be Wagner's electrochemical potential gradient-induced migration of defect species across the iodide layer.