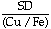PYROMETALLURGY
Synthesis of Neodymium Aluminide by Aluminothermic Reduction of Neodymium Oxide
A. BISWAS, I.G. SHARMA, G.B. KALE, and D.K. BOSE
Neodymium aluminide was synthesized from Nd2O3 under vacuum by aluminothermic reduction in an induction furnace. Although the standard free energy change associated with the reduction of Nd2O3 by Al is positive, due to the formation reaction of the intermetallic compound, it was possible to drive the reaction in the forward direction in the presence of excess Al. An attempt was also made to reduce Nd2O3 by Al in the thermit process in the presence of the Fe2O3-Al thermit mixture so as to make the reaction self-sustaining. A comparison between these two processes was made with respect to the reduction of Nd2O3 by Al. Differential thermal analysis (DTA) was used to study the reaction process. The product was characterized by x-ray diffraction (XRD), x-ray fluoroscence (XRF), electron probe microanalysis (EPMA), and optical metallography (OM).
Surface Interactions between Fayalite Slags and Synthetic Spinels and Solid Solutions
J.R. DONALD, J.M. TOGURI, and C. DOYLE
To obtain a better understanding of the complex corrosion mechanisms occurring at the interface, the surface and interfacial properties between fayalite-type slags and homogeneous, synthetic spinels and solid solutions of these spinels were investigated. These oxides represent the conventional refractory components. The sessile drop technique incorporating high-temperature X-ray radiography was employed for this purpose. The experimental temperature was 1200°C and the oxygen potential was 10-9 atm controlled by CO/CO2 gas mixture. The contact angles between the solid substrates and molten silica-rich fayalite slag ranged from 0 deg for MgFe2O4 to 23 deg for MgAl2O4. When iron-rich slags were employed, the contact angles ranged from 15 deg for MgCr2O4 to 22 deg for MgAl2O4. The interfacial reactions between the slags and the various spinel materials and the dissolution of the solids into the slags are discussed.
Reactive Phosphide Inclusions in Commercial Ferrosilicon
Q.C. HORN, R.W. HECKEL, and C.L. NASSARALLA
The goal of this work was to determine the origin of phosphine gas (PH3), which has been reported to be generated from wet, commercial ferrosilicon alloys containing ~75 wt pct Si. Based on previous work, it is suspected that PH3 evolves when phosphides present within the alloy react with atmospheric moisture or water. Reactive phosphides have been identified in synthetic ferrosilicon alloys, which contain higher amounts of phosphorus than are typically present in commercial alloys. Therefore, reactive phosphides in commercial FeSi75 alloys are expected to be important to the evolution of PH3 from these alloys. To identify the role of reactive phosphides in the evolution of PH3 from commercial FeSi75 alloys, the microstructures of four commercial and two synthetic FeSi75 ferrosilicon alloys were investigated. Reactive phosphides were observed in each of the commercial alloys and characterized with respect to composition, morphology, and location within the microstructure. The phosphides observed in all of the commercial alloys contained aluminum, calcium, and magnesium. The phosphides had inclusion-like morphologies and were located on the silicon/ (high-temperature FeSi2) interfaces at microcracks. The microstructural features observed support the hypothesis that atmospheric moisture penetrates ferrosilicon, reacting with the phosphide inclusions to produce PH3. A possible mechanism describing the spontaneous crumbling sometimes observed in ferrosilicon alloys is also presented.
(high-temperature FeSi2) interfaces at microcracks. The microstructural features observed support the hypothesis that atmospheric moisture penetrates ferrosilicon, reacting with the phosphide inclusions to produce PH3. A possible mechanism describing the spontaneous crumbling sometimes observed in ferrosilicon alloys is also presented.
Formation Mechanism of LaNi5 in the Reduction-Diffusion Process
T. TANABE and Z. ASAKI
To clarify the mechanism of the formation of LaNi5 by the reduction-diffusion (RD) process, two kinds of experiments were carried out: (1) briquets consisting of La2O3, CaH2, and Ni wires were heated at 1300 K in the RD experiment; and (2) Ni wire was immersed into an Ni-La-Ca melt with a composition on the Ni-side liquidus surface at 1300 K. On the surface of Ni, LaNi5 grew and the formation of CaNi5 was also observed between the LaNi5 and Ni in the RD reaction; both layers grew in accordance with a parabolic rate law. Even in the reaction of Ni wire with an Ni-La-Ca ternary melt of low Ca concentrations, CaNi5 initially grew on the nickel surface before LaNi5 was formed, and the Ca in CaNi5 was replaced with La to form LaNi5. The reason for the initial formation of CaNi5 was discussed using molecular orbital calculations. These calculations show that the preferential and temporary formation of CaNi5 and the final production of LaNi5 can be explained by the electronic structures of Ni alloys containing Ca or La and those of the compounds, CaNi5 and LaNi5, respectively.
Communication: Removal of Copper from Carbon-Saturated Iron with an Aluminum Sulfide/Ferrous Sulfide Flux
A. COHEN AND M. BLANDER
TRANSPORT PHENOMENA
Particle Suspension in (Air-Agitated) Pachuca Tanks
G.G. ROY, R. SHEKHAR, and S.P. MEHROTRA
Particle suspension is an important parameter in the design of an energy-efficient Pachuca tank. Unfortunately, very little attention has been focused on the suspension behavior of air-agitated Pachucas. In the present investigation, therefore, extensive experiments have been carried out in three laboratory-scale Pachuca tanks to examine the effect of design and operating parameters, as well as scale-up, on particle suspension. A mathematical model that combines the Bernoulli's equation and the theory of transport of particles in the horizontal flow of a liquid has been developed to predict the critical gas velocity for particle suspension in Pachuca tanks. Some important results, crucial to the design and scale-up of Pachuca tanks, have emerged. Full-center-column (FCC) Pachuca tanks with a draft tube-to-tank diameter ratio (Dd/Dt) on the order of 0.1 are found to be energetically more efficient in suspending particles than free-air-lift (FAL) and stub-column (SC) Pachuca tanks. It is also observed that taller tanks require lower air flow rates for particle suspension than shallower tanks. Finally, it is explained why industrial Pachuca tanks operate at lower air velocities than laboratory-scale tanks.
PHYSICAL CHEMISTRY
Thermodynamic Estimation on the Reduction Behavior of Iron-Chromium Ore with Carbon
MITSUTAKA HINO, KEN-ICHI HIGUCHI, TETSUYA NAGASAKA, and SHIRO BAN-YA
The thermodynamics for reduction of iron-chromium ore by carbon is discussed. The thermodynamic properties of iron-chromium ore were evaluated from our previous work on the activities of constituents in the FeO · Cr2O3-MgO · Cr2O3-MgO · Al2O3 iron-chromite spinel-structure solid solution saturated with (Cr, Al)2O3, and those of the Fe-Cr-C alloy were estimated by a sublattice model. The stability diagrams were drawn for carbon reduction of pure FeO · Cr2O3, (Fe0.5Mg0.5)O · (Cr0.8Al0.2)2O3 iron-chromite solid solution, and South African iron-chromium ore. The evaluated stability diagrams agreed well with the literature data. It was concluded that the lowest temperature for reduction of FeO · Cr2O3 in the iron-chromium ore was 1390 K and a temperature higher than 1470 K would be necessary to reduce Cr2O3 in MgO · (Cr,Al)2O3 in the prereduction process of iron-chromium ore. The composition of liquid Fe-Cr-C alloy in equilibrium with iron-chromium ore was also estimated under 1 atm of CO at steelmaking temperature. The predicted metal composition showed reasonable agreement with the literature values.
Assessment of the Fe-Ti System
STEFAN JONSSON
The literature on the Fe-Ti system is reviewed and the thermodynamic description of the system is reassessed checking the ternary extrapolations in the Fe-Ti-C and Fe-Ti-N systems. The reproduction of the thermochemical and phase diagram information is presented through a series of figures and tables.
Assessment of the Fe-Ti-C System, Calculation of the Fe-Ti-N System, and Prediction of the Solubility Limit of Ti(C,N) in Liquid Fe
STEFAN JONSSON
The literature on the Fe-Ti-C, Fe-Ti-N, and Fe-Ti-C-N systems is reviewed and the experimental information is reproduced by thermodynamic calculations. In the Fe-Ti-C system, interaction parameters are evaluated for the liquid and fcc phases. In the Fe-Ti-N and Fe-Ti-C-N systems, on the other hand, no interactions are evaluated because of the very low solubility of TiN and Ti(C,N) in liquid Fe, bcc(Fe), and fcc(Fe). The Fe-Ti-C and Fe-Ti-N phase diagrams are presented through a series of sections and projections, and the solubility limit of Fe(Ti,N) in liquid Fe is calculated.
The Kinetics and Mechanism of the Pyrite-to-Pyrrhotite Transformation
J.M. LAMBERT, JR., G. SIMKOVICH, and P.L. WALKER, JR.
The kinetics of the transformation of pyrite to pyrrhotite have been investigated. The study was performed using thermogravimetric analysis over the temperature range of 620 to 973 K in atmospheres of H2, He, Ar, and in vacuo over a wide range of pressures: 0.20 Pa to 4.24 MPa. Based on the kinetic results, a mechanistic picture of the various steps exerting control over the transformation is proposed. The thermal decomposition proceeds via a two-step, consecutive process. The rate-controlling step is the desorption of sulfur vapor from the surface. The presence of H2 introduces different rate-controlling steps into the sequence, providing the H2 exists at a pressure sufficiently high to suppress the rate of thermal decomposition. Rates at which the H2 reduction occurs with pyrite samples from different sources depends upon the samples' impurity level and the extent to which various crystallographic faces are exposed.
Thermodynamic Assessment of Liquid Fe-Mn-C System
YOUNG E. LEE
The lattice site ratio model proposed by Chipman describes the activity of the interstitial solutes in the alloy systems in terms of the ratio of filled to unfilled sites as a concentration parameter. Chipman and co-workers successfully applied the model to describe the solution behavior of C and S in the various alloy systems. The meaning of the lattice for the liquid system is not same as that for the solid system, as it does not retain the rigidity of a solid crystalline structure and a long-range order in the atomic arrangement. Still, the liquid system maintains a short-range order with a similar structure to that of the close-packed solid system, and the application of the lattice site ratio model to the liquid system is justified. The present study assessed the thermodynamic properties of the liquid Fe-Mn-C system with the use of the lattice site ratio model. It was found that the solution properties of the ternary Fe-Mn-C system can be represented as linear combinations of those of the constituent binary systems if they are defined in the lattice site ratio model. This is not the case when the solution properties are given in the mole fraction coordinate. The lattice site ratio model reproduced closely the experimentally determined solubility of C and the activities of C and Mn in the Fe-Mn-C system.
Formation of Hexavalent Chromium by Reaction between Slag and Magnesite-Chrome Refractory
Y. LEE and C.L. NASSARALLA
The goal of this work was to understand how Cr6+ formation is affected by the interaction between chromite phases present in magnesite-chrome refractory and different slag compositions. In addition, the formation of Cr6+ as a function of chromite particle size and cooling rate due to the chromite phase/slag interaction was investigated. The following slag compositions were studied: calcium aluminate, calcium aluminum silicate, and calcium silicate. It was found that the presence of uncombined CaO in the calcium aluminate slags is responsible for a higher yield of Cr6+. However, the replacement of Al2O3 by SiO2 in calcium aluminate slags decreases the formation of Cr6+. SiO2 reacts with CaO to form stable 2CaO·Al2O3·SiO2 and CaO·SiO2 phases, consequently decreasing the amount of uncombined CaO available to react with the chromite phase to form Cr6+. Moreover, the content of Cr6+ is decreased by increasing chromite particle size and increasing the cooling rate of magnesite-chrome refractory.
The Influence of Basicity on the Solubility of Platinum in Oxide Melts
SHIGEKO NAKAMURA, KOKORO IWASAWA, KAZUKI MORITA, and NOBUO SANO
The solubility of platinum in molten BaO-CuOx, BaO-MnOx, CaOsatd-SiO2-FeOx, KO0.5-SiO2, NaO0.5-SiO2, and NaO0.5-PO2.5 fluxes has been measured in order to seek a measure of the basicity of highly basic fluxes containing transition metal ions and to clarify the chemical behavior of platinum in those melts. The solubility of platinum increases with increasing content of basic oxide in highly basic fluxes, suggesting that it may be a good indicator of the basicity of highly basic fluxes containing transition metal ions. The solubility of platinum in the KO0.5-SiO2, NaO0.5-SiO2, and NaO0.5-PO2.5 melts has a minimum value, and it is suggested that a platinum is amphoteric: it exists as a platinum cation in an acidic flux and does as a platinate ion in a basic flux.
Equilibrium of Calcium Vapor with Liquid Iron and the Interaction of Third Elements
BO SONG and QIYONG HAN
The dissolution equilibrium of calcium vapor in liquid iron was carried out at 1873 K in a two-temperature zone furnace using a vapor-liquid equilibration method. A sealed Mo reaction chamber and a self-made CaO crucible were used in this study. The thermodynamic parameters obtained are as follows.
For reaction Ca (g) = [Ca],
ln K = 4.27 - 15,040/T  G° = 125,000 - 35.5T J/mole
G° = 125,000 - 35.5T J/mole
The relation between dissolved calcium in liquid iron and calcium vapor can be expressed as:
[pct Ca] = 0.0235 PCa/(l - 0.305 PCa
The interaction parameters of third elements on calcium determined at 1873 K are as follows:
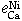 = -0.097 ± 0.007,
= -0.097 ± 0.007,
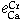 = -0.18 ± 0.02,
= -0.18 ± 0.02,
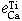 = -0.13 ± 0.009,
= -0.13 ± 0.009,
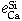 = -0.11 ± 0.005,
= -0.11 ± 0.005,
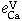 = -0.15 ± 0.015,
= -0.15 ± 0.015,
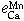 = -0.10 ± 0.013,
= -0.10 ± 0.013,
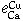 = -0.023 ± 0.003,
= -0.023 ± 0.003,
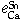 = -0.026 ± 0.003,
= -0.026 ± 0.003,
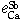 = -0.043 ± 0.005,
= -0.043 ± 0.005,
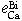 = -0.15 ± 0.035
= -0.15 ± 0.035
A Novel Method for the Determination of the Hydrogen Solubility in Aluminum and Aluminum Alloy Melts
A. SZOKEFALVI-NAGY, L. STOJANOVA, and E. FROMM
The CHAPEL method, which was developed for the direct and continuous determination of the hydrogen activity in aluminum melts by measuring the equilibrium hydrogen pressure, has been modified for the determination of the hydrogen solubility in aluminum and aluminum alloy melts. The change of the hydrogen equilibrium pressure due to addition or removal of a given amount of hydrogen from a melt of known mass yields directly the Sieverts constant. Such experiments provide reliable data only if no additional gas exchange takes place between the melt and the gas phase. In the present work, a quasi-impermeable interface between the melt and the surrounding hydrogen atmosphere has been realized by maintaining the hydrogen pressure above the melt continuously at the level of the hydrogen equilibrium pressure. By this technique, which is performed by a relatively simple experimental setup, fast determination of the hydrogen solubility is possible. The main advantage of this novel method is the fact that it can be applied also for aluminum alloys without protective oxide layer on the surface. Preliminary results on pure aluminum and Al-Cu alloy melts show good agreement with the data obtained by the classical method of Sieverts that is not well suited for routine determinations on a wide range of alloy composition and temperature since it is very time-consuming. The method can also be applied for the investigation of other metal and alloy melts.
Henrian Activity Coefficient of Pb in Cu-Fe Mattes and White Metal
TOM ZHONG and DAVID C. LYNCH
A transpiration method was used to evaluate the Henrian activity coefficient of Pb (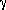 °Pb) in Cu-Fe mattes and white metal. Values for the activity coefficient of Pb (
°Pb) in Cu-Fe mattes and white metal. Values for the activity coefficient of Pb (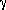 Pb) have been evaluated as a function of the Cu/Fe molar ratio from 1 to
Pb) have been evaluated as a function of the Cu/Fe molar ratio from 1 to  , as a function of the sulfur deficiency (defined as SD = XS - 1/2XCu - XFe, where Xl is the mole fraction of the ith species) from -0.02 to +0.02, and at temperatures between 1493 and 1573 K. Analysis of
, as a function of the sulfur deficiency (defined as SD = XS - 1/2XCu - XFe, where Xl is the mole fraction of the ith species) from -0.02 to +0.02, and at temperatures between 1493 and 1573 K. Analysis of 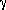 Pb as a function of the trace element concentration reveals that the activity coefficient is independent of Pb content at weight percents less than 0.2. Dependence of
Pb as a function of the trace element concentration reveals that the activity coefficient is independent of Pb content at weight percents less than 0.2. Dependence of 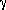 Pb on temperature was found to be slight, and as such, comparison of data obtained by other investigators at 1473 K was possible. Agreement in the data is excellent, and all the data have been used to generate the empirical equation
Pb on temperature was found to be slight, and as such, comparison of data obtained by other investigators at 1473 K was possible. Agreement in the data is excellent, and all the data have been used to generate the empirical equation
log
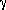 °Pb = 0.609 - 40.0SD -
°Pb = 0.609 - 40.0SD -
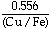 - 12.9SD2 +
- 12.9SD2 +
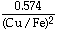 + 25.0
+ 25.0
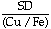
that is valid over the temperature range from 1473 to 1573 K. The experimental results suggest that in high sulfur content melts, lead is stabilized as PbS. The results also reveal that free copper, in sulfur deficient mattes, tends to stabilize Pb, but to a lesser extent than that experienced with excess sulfur in high sulfur melts. Failure to account for sulfur loss can lead to a significant error. This article also presents a method whereby sulfur loss during experiments can be accounted for in computing activity coefficients.
SOLIDIFICATION
A Correlation to Describe Interfacial Heat Transfer during Solidification Simulation and Its Use in the Optimal Feeding Design of Castings
R.W. LEWIS and R.S. RANSING
It is known from experimental data that for pure aluminum castings manufactured via the gravity die casting process, the interfacial heat-transfer coefficient can vary in the range 500 to 16,000 W/m2 K. These coefficients are of significant importance for the numerical simulation of the solidification process. The experimentally determined variation of interfacial heat-transfer coefficients with respect to time has been recalculated to highlight the variation with respect to casting temperature at the interface. This variation was observed to be of an exponential nature. Also, the pattern of variation was found to be similar in all the experimental results. It has been found that all these patterns of interfacial heat-transfer coefficient variation can be matched by a unique equation that has been proposed as a correlation to model the metal-mold interfacial heat transfer. The benefit of this correlation is in its ability to approximate the combined effects of geometry variation, insulation, chills, die coatings, air gap formation, etc. during the numerical simulation and its use in the optimal design of heat transfer at the metal-mold interface.
SOLID STATE REACTIONS
Mechanically Activated Reduction of Nickel Oxide with Graphite
H. YANG and P.G. McCORMICK
The reduction of nickel oxide with graphite during ball milling at both ambient and elevated temperatures was investigated using X-ray diffraction (XRD), simultaneous thermogravimetry and differential thermal analysis (TG/DTA), and electron microscopy. It was found that milling at ambient temperature did not result in the reduction of nickel oxide to nickel. However, milling significantly reduced the critical reaction temperature for the reduction, from 1350 K for the unmilled sample to ~650 K for samples milled for 12 hours or longer. This reduction in reaction temperature is rationalized in terms of the microstructural refinement observed in the milled samples. The reduction of nickel oxide to nickel was observed to occur at elevated temperatures during milling. The thermodynamics and kinetics of the reduction reaction are discussed.
MATERIALS PROCESSING
Synthesis of Ultrafine Particles of Intermetallic Compounds by the Vapor-Phase Magnesium Reduction of Chloride Mixtures: Part I. Titanium Aluminides
H.Y. SOHN and S. PALDEY
A new chemical synthesis process for the preparation of intermetallic compounds has been developed. It involves the vapor-phase reduction of mixtures of constituent metal chlorides by magnesium vapor to produce intermetallic compounds in the form of fine powder. The advantages of the process include (a) the use of inexpensive raw materials, (b) low reaction temperatures, and (c) products in the form of fine particles. Part I describes the synthesis of titanium aluminide particles by this method, whereas Part II presents the synthesis of nickel aluminides particles. Although nickel aluminides can also be prepared by the hydrogen reduction of nickel chloride and aluminum chloride vapor mixtures, titanium aluminides cannot be produced by hydrogen reduction because of unfavorable thermodynamics. The effect of AlCl3/TiCl3 partial pressure ratio on the formation of different titanium aluminides was studied. A two-phase mixture containing 80 mol pct of TiAl + 20 mol pct of TiAl3 formed at an AlCl3/TiCl3 ratio of 10. The amount of TiAl3 was maximized to 72 mol pct at an AlCl3/TiCl3 ratio of 16. The maximum conversion of the limiting chloride TiCl3 was 94 pct. The product particles were very fine in the size range of 0.2 to 0.3 µm.
Synthesis of Ultrafine Particles of Intermetallic Compounds by the Vapor-Phase Magnesium Reduction of Chloride Mixtures: Part II. Nickel Aluminides
H.Y. SOHN and S. PALDEY
The new chemical synthesis process developed in this laboratory for the preparation of the fine powders of intermetallic compounds by the vapor-phase reduction of mixtures of constituent metal chlorides by magnesium vapor, described in Part I for titanium aluminides, was applied to the synthesis of nickel aluminide particles. NiAl, NiAl3, and Ni2Al3 were formed by reducing mixtures of NiCl2 and AlCl3 vapors. The effect of the partial pressure ratios AlCl3/NiCl2 and Mg/NiCl2 on the formation of nickel aluminides was studied. The maximum content of NiAl obtained was 98 mol pct. At the AlCl3/NiCl2 ratio of 19, a two-phase mixture of 17 mol pct of NiAl + 83 mol pct of NiAl3 was produced. The product particles were very fine in the size range of 0.1 to 0.2 µm.
Influence of Melt Carbon and Sulfur on the Wetting of Solid Graphite by Fe-C-S Melts
C. WU and V. SAHAJWALLA
The interfacial phenomena between carbonaceous materials such as graphite, coke, coal, and char and Fe-C-S melts are important due to the extensive use of these materials in iron processing furnaces. However, the understanding of the interfacial phenomena between these kinds of carbonaceous materials and molten iron alloys is far from complete. In this study, graphite was selected as the solid carbonaceous material because its atomic structure has been well established. The sessile drop method was adopted in this investigation to measure the contact angle between solid graphite and molten iron and to study the interfacial phenomena. The influence of carbon and sulfur content in Fe-C-S melts on the wettability of solid graphite has been investigated at 1600°C. The melt carbon content was in the range of 0.13 to 2.24 wt pct, and the melt sulfur content was in the range of 0.05 to 0.37 wt pct. X-ray energy-dispersive spectrometer (EDS) analysis was conducted on an HITACHI S-4500 scanning electron microscope to detect composition distribution at the interfacial region. It was found that contact of solid graphite with Fe-C-S melts will result in a nonequilibrium reactive wetting. It involved carbon transfer from the solid to the liquid and iron transfer from the liquid to the solid. The Fe-C-S melts exhibited relatively poor wetting when the reactions were absent. The mass transfer between solid graphite and Fe-C-S melts was observed to strongly enhance the wetting phenomena. It is proposed that the decrease of system free energy corresponding to the mass transfer reactions strongly influences the formation of the interface region and results in the progressive spreading of the wetting line. The composition and thickness of the graphite/iron interfacial layer was dependent on the intensity of mass transfer across the interface. The resulting change in the interfacial energy 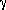 ls is a strong function of mass transfer, and it varies in accordance with time of contact. The influence of carbon content on the wetting phenomena could only be seen at in the initial stages, whereas the influence of sulfur on the wettability was found when the system approached equilibrium. Therefore, the interfacial tension in its equilibrium condition at the graphite/Fe-C-S melt interface was determined only by the extent of sulfur adsorption at this interface.
ls is a strong function of mass transfer, and it varies in accordance with time of contact. The influence of carbon content on the wetting phenomena could only be seen at in the initial stages, whereas the influence of sulfur on the wettability was found when the system approached equilibrium. Therefore, the interfacial tension in its equilibrium condition at the graphite/Fe-C-S melt interface was determined only by the extent of sulfur adsorption at this interface.
SURFACE TREATMENT
The Effect of Iron Oxide as an Inhibition Layer on Iron-Zinc Reactions during Hot-Dip Galvanizing
C.E. JORDAN and A.R. MARDER
A study was conducted on the effect of a uniform oxide layer on the galvanizing reaction in 0.20 wt pct Al-Zn and pure Zn baths at 450°C. In the 0.20 wt pct Al-Zn bath, poor wettability of the oxide layer was observed. No significant liquid Zn penetration of the oxide occurred and, therefore, attack of the steel substrate to form localized Fe-Zn growth did not occur. It was found that the iron oxide acted as a physical barrier or inhibition layer in the pure Zn bath, similar to the Fe2Al5 inhibition layer that forms at the steel interface in Al-Zn baths. The inhibition effect of the oxide in the pure Zn bath was temporary, since cracks and other macrodefects in the oxide acted as fast diffusion paths for Zn. Localized Fe-Zn growth (outbursts) formed at the steel/coating interface, and the number of outbursts was generally inversely proportional to the oxide layer thickness at constant immersion times. Increased immersion time for a constant oxide layer thickness led to an increase in the number of outbursts. These results simulate the diffusion short circuit mechanisms for Fe2Al5 inhibition layer breakdown in Al-containing Zn baths.
MATHEMATICAL MODELING
Modeling the Heat Flow to an Operating Sirosmelt Lance
C.B. SOLNORDAL, F.R.A. JORGENSEN, and R.N. TAYLOR
A mathematical model of the heat flow to a Sirosmelt lance is presented, which predicts lance wall and air temperatures and the thickness of the slag layer on the lance. By measuring the distribution of wall temperature and slag thickness on an operating Sirosmelt lance, the model was used to determine both the heat-transfer coefficient between the vessel contents and the lance and the thermal conductivity of the slag layer. The slag layer thermal conductivity was found to be within the range of 0.5 to 1.1 W m-1 K-1, while the outside heat-transfer coefficient varied from 80 to 150 W m-2 K-1, both of which are smaller than quoted in the literature for metal/slag systems. The discrepancy was attributed primarily to the large quantities of combustion gases that envelop the lance and reduce convection and conduction from the melt to the lance. Other factors causing low thermal conductivity and a low heat-transfer coefficient include the thermal resistance at the slag/lance interface and the mushy region on the outside of the slag layer.
Communication: A Monte Carlo Approach for Simulation of Heat Flow in Sand and Metal Mold Castings (Virtual Mold Modeling)
LAURENTIU NASTAC
Direct questions about this or any other Metallurgical and Materials Transactions page to mettrans@andrew.cmu.edu.

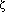 (high-temperature FeSi2) interfaces at microcracks. The microstructural features observed support the hypothesis that atmospheric moisture penetrates ferrosilicon, reacting with the phosphide inclusions to produce PH3. A possible mechanism describing the spontaneous crumbling sometimes observed in ferrosilicon alloys is also presented.
(high-temperature FeSi2) interfaces at microcracks. The microstructural features observed support the hypothesis that atmospheric moisture penetrates ferrosilicon, reacting with the phosphide inclusions to produce PH3. A possible mechanism describing the spontaneous crumbling sometimes observed in ferrosilicon alloys is also presented.
 G° = 125,000 - 35.5T J/mole
G° = 125,000 - 35.5T J/mole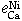 = -0.097 ± 0.007,
= -0.097 ± 0.007,
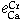 = -0.18 ± 0.02,
= -0.18 ± 0.02,
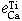 = -0.13 ± 0.009,
= -0.13 ± 0.009, 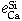 = -0.11 ± 0.005,
= -0.11 ± 0.005,
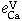 = -0.15 ± 0.015,
= -0.15 ± 0.015,
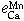 = -0.10 ± 0.013,
= -0.10 ± 0.013, 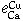 = -0.023 ± 0.003,
= -0.023 ± 0.003,
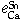 = -0.026 ± 0.003,
= -0.026 ± 0.003, 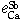 = -0.043 ± 0.005,
= -0.043 ± 0.005,
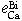 = -0.15 ± 0.035
= -0.15 ± 0.035 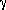 °Pb) in Cu-Fe mattes and white metal. Values for the activity coefficient of Pb (
°Pb) in Cu-Fe mattes and white metal. Values for the activity coefficient of Pb ( , as a function of the sulfur deficiency (defined as SD = XS - 1/2XCu - XFe, where Xl is the mole fraction of the ith species) from -0.02 to +0.02, and at temperatures between 1493 and 1573 K. Analysis of
, as a function of the sulfur deficiency (defined as SD = XS - 1/2XCu - XFe, where Xl is the mole fraction of the ith species) from -0.02 to +0.02, and at temperatures between 1493 and 1573 K. Analysis of 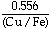 - 12.9SD2 +
- 12.9SD2 +
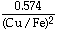 + 25.0
+ 25.0