 |
49 (6) (1997), pp. 15-19. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
 |
49 (6) (1997), pp. 15-19. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
| CONTENTS |
|---|
|
|
In the 1940s, it was a common belief that atomic diffusion took place via a direct exchange or ring mechanism that indicated the equality of diffusion of binary elements in metals and alloys. However, Ernest Kirkendall first observed inequality in the diffusion of copper and zinc in interdiffusion between brass and copper. This article reports how Kirkendall discovered the effect, now known as the Kirkendall Effect, in his short research career.
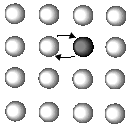
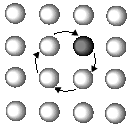
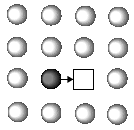
Is atomic diffusion possible in a solid metal where atoms arrange themselves regularly? The answer is yes. Although atomic diffusion in solids is far slower than that in gases and liquids, diffusion does take place. The diffusion in solids is clearly related to various processes such as recrystallization, precipitation, and oxidation. Such study of the diffusion in solids was initiated just 100 years ago when Sir Roberts-Austen1 discovered the diffusion phenomenon of gold in solid lead in 1896. For a long time afterward, people believed that atomic diffusion occurred by a direct exchange mechanism or a ring mechanism in metallic crystals (Figure 1).
 |
 |
 |
| a | b | c |
| Figure 1. The atomic diffusion mechanism showing (a) a direct exchange mechanism, (b) ring mechanism, and (c) vacancy mechanism. | ||
When the International Conference on Diffusion in Metals and Alloys was held in 1988 in Balatonfured, Hungary, S. Rothman of Argonne National Laboratory (and also the editor of Journal of Applied Physics) told me of an episode during the discovery of the new effect by Kirkendall. Kirkendall's idea had been criticized by many researchers, but he kept trying until his interpretation had almost been accepted. When a search committee was set up to determine Kirkendall's possible promotion to associate professorship, one of the referees, R.F. Mehl of the Carnegie Institute of Technology (an authority on diffusion), rejected Kirkendall's promotion. Shortly thereafter, Kirkendall gave up his academic career and took a job as secretary of the American Institute of Mining and Metallurgical Engineers (AIME). Although the story related by Rothman was certainly interesting to me, my boss at that time (H.B. Huntington, Rensselaer Polytechnic Institute) told a different version of the story, and I wondered which version was right.
Fifty years have passed since Dr. Kirkendall finished his academic career. Fortunately, several friends informed me that he was still in good health, and I succeeded in calling him when I was staying in Canada in 1993. I was so excited to talk with him; he politely replied to my various inquiries and kindly suggested I visit him at his home "since such a telephone conversation is not sufficient." At that time, I could not visit him because I had to leave Canada to return to Japan within a few days. Two months later, I finally visited his home and interviewed him. Based on that meeting, this article reports how Kirkendall discovered the Kirkendall Effect in a short research career in which he produced only three papers.2,4
To illustrate the importance of his discovery, the Kirkendall Effect Symposium on Interdiffusion and Phenomena that Depend on Net Vacancy Flows was held during the TMS Fall Meeting in October 1991. I heard that this was successful meeting, attended by authors such as professors Turnbull, Balluffi, Huntington, Cahn, and Heumann, who had contributed to lattice defects and diffusion research in the period following the discovery of the Kirkendall Effect.
 -brass and
-brass and  -brass resulting from the cooling of
-brass resulting from the cooling of  -brass alloy. For his research topic, he selected diffusion in brass, which was a combination of those two interests. Although such research had already been done by several researchers, most were nothing more than qualitative discussions, and none of the research could elucidate the key questions until quantitative evaluation and discussion had been achieved. Thus, Kirkendall wanted to measure the diffusion coefficients of copper and zinc in
-brass alloy. For his research topic, he selected diffusion in brass, which was a combination of those two interests. Although such research had already been done by several researchers, most were nothing more than qualitative discussions, and none of the research could elucidate the key questions until quantitative evaluation and discussion had been achieved. Thus, Kirkendall wanted to measure the diffusion coefficients of copper and zinc in  -brass quantitatively with high accuracy by using a "new method." The result was his D.Sc. disertation,2 which reported zinc diffusivities in brass at three different temperatures.
-brass quantitatively with high accuracy by using a "new method." The result was his D.Sc. disertation,2 which reported zinc diffusivities in brass at three different temperatures.
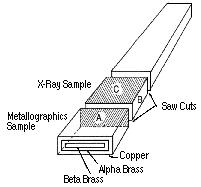
|
| Figure 2. A schematic of the specimen used for the study of the first paper.2 |
 -brass plate-shaped specimen (100 mm in length and about 3 mm in thickness) was first mirror-polished; it was then electroplated with 250 mm thickness of copper (Figure 2). The specimens were annealed in vacuum at 723 K for about 10 ks to make hydrogen desorb from the specimens. Diffusion anneals were carried out in a muffle electric furnace at a specific temperature and for a specific duration. After water quenching, a part of the specimen was cut off, while the remainder was used for successive diffusion anneals. The metallographic observation of the polished surface A was performed with an optical microscope. X-ray diffraction was taken from the polished surface B after each sectioning step of 25-75 µm thickness to measure the lattice parameters, which were then converted to determine the zinc concentration in
-brass plate-shaped specimen (100 mm in length and about 3 mm in thickness) was first mirror-polished; it was then electroplated with 250 mm thickness of copper (Figure 2). The specimens were annealed in vacuum at 723 K for about 10 ks to make hydrogen desorb from the specimens. Diffusion anneals were carried out in a muffle electric furnace at a specific temperature and for a specific duration. After water quenching, a part of the specimen was cut off, while the remainder was used for successive diffusion anneals. The metallographic observation of the polished surface A was performed with an optical microscope. X-ray diffraction was taken from the polished surface B after each sectioning step of 25-75 µm thickness to measure the lattice parameters, which were then converted to determine the zinc concentration in  -brass (Figure 2).
-brass (Figure 2).
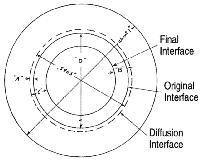
After diffusion anneals, an  -brass phase layer grows on both sides of the bonding interface of copper and
-brass phase layer grows on both sides of the bonding interface of copper and 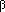 -brass (Figure 3). The
-brass (Figure 3). The  -brass layer inside the interface consists of large columnar grains so that the
-brass layer inside the interface consists of large columnar grains so that the  /
/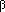 phase boundary is clear. On the other hand, since the copper-rich
phase boundary is clear. On the other hand, since the copper-rich  -brass phase outside the interface has the same crystal structure as the copper phase, the boundary is not distinct.
-brass phase outside the interface has the same crystal structure as the copper phase, the boundary is not distinct.
Kirkendall told me, "The quantitative analysis of the work was possible owing to two points—the  -brass phase in between the original (bonding) interface and the final
-brass phase in between the original (bonding) interface and the final  /
/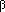 interface always grows uniformly in thickness, and the
interface always grows uniformly in thickness, and the  /
/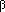 interface can be observed clearly with the optical microscope. If the
interface can be observed clearly with the optical microscope. If the  /
/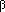 interface and the columnar grains formed on the inside of the original copper
interface and the columnar grains formed on the inside of the original copper 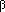 -brass interface had not been distinct, the Kirkendall Effect would not have been discovered. Thus, I was lucky to choose the system of copper and brass originally." He measured the concentration profiles of copper diffusion in
-brass interface had not been distinct, the Kirkendall Effect would not have been discovered. Thus, I was lucky to choose the system of copper and brass originally." He measured the concentration profiles of copper diffusion in  -brass phase surrounded by both the original and the
-brass phase surrounded by both the original and the  /
/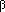 interfaces.
interfaces.
 |
 |
||
| a | b | e | f |
 |
 |
||
| c | d | g | h |
| Figure 3. Metallographic observation (magnified 200x) at (a) as bonded, (b) one hour, (c) one hour, (d) four hours, (e) ten hours, (f) 24 hours, (g) 96 hours, and (h) 96 hours. | |||
 DZn)." The Boltzmann-Matano interface fixes two equal areas on the concentration profile c(x); it is the plane through which equal amounts of material have moved in positive and negative directions.
DZn)." The Boltzmann-Matano interface fixes two equal areas on the concentration profile c(x); it is the plane through which equal amounts of material have moved in positive and negative directions.
At that time, it was common belief that atomic diffusion took place via a direct exchange or ring mechanism. None of the researchers proposed other mechanisms. Thus, according to his advisor's suggestion, "I preferred to explain the movement of the original interface as due to volume change between  - and
- and 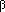 -brass;
-brass;  -brass has fcc close-packed structure while
-brass has fcc close-packed structure while 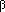 -brass has bcc less close-packed structure." Moreover, he concluded that on the assumption of the equality of the diffusivities of copper and zinc, diffusion of these elements must have occurred by the ring mechanism in which four or more atoms participate. He recalled that if he had insisted on the inequality of these diffusivities to interpret the original boundary migration, Upthegrove would have opposed Kirkendall's Sc.D. thesis defense.
-brass has bcc less close-packed structure." Moreover, he concluded that on the assumption of the equality of the diffusivities of copper and zinc, diffusion of these elements must have occurred by the ring mechanism in which four or more atoms participate. He recalled that if he had insisted on the inequality of these diffusivities to interpret the original boundary migration, Upthegrove would have opposed Kirkendall's Sc.D. thesis defense.
|
| Figure 4. A schematic of a disc-shaped specimen used for the second paper.3 |
 -ray was the most effective for brass samples, an x-ray tube with a cobalt target was produced. Furthermore, he obtained a high-voltage power supply for Röntgen photography from a local dentist, and set up the x-ray diffraction apparatus.
-ray was the most effective for brass samples, an x-ray tube with a cobalt target was produced. Furthermore, he obtained a high-voltage power supply for Röntgen photography from a local dentist, and set up the x-ray diffraction apparatus.
Disc-shaped specimens of 15 mm in diameter were used (Figure 4). Muntz metal with 60.6% copper, 0.1% total impurities, and the balance in zinc was used for the specimen, whose surface was electroplated with copper 5.12 mm in thickness. After the diffusion anneals, metallographic observation of a section of the specimen was done with the optical microscope, and the displacement between the original interface and the  /
/ phase boundary was measured. After each removal of a 200-250 µm thick layer by a lathe, x-rays were directed onto the specimen to measure the surface lattice parameter, which yielded zinc concentration profiles in the specimen.
phase boundary was measured. After each removal of a 200-250 µm thick layer by a lathe, x-rays were directed onto the specimen to measure the surface lattice parameter, which yielded zinc concentration profiles in the specimen.
Figure 5 shows metallographic observations after successive diffusion anneal at 1,053 K up to 2,523.6 ks. As seen in the figure, a layer with  -brass phase was formed on both sides of the original interface. In particular, the
-brass phase was formed on both sides of the original interface. In particular, the  -brass phase on the
-brass phase on the  -brass side consisted of large columnar grains, and the
-brass side consisted of large columnar grains, and the  /
/ interface was very clear because of the different crystal structures in
interface was very clear because of the different crystal structures in  -brass (fcc) and
-brass (fcc) and 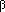 -brass (bcc). On the other hand, in the copper-rich
-brass (bcc). On the other hand, in the copper-rich  -brass phase, neither a distinct difference in the grain size nor a clear
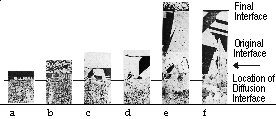
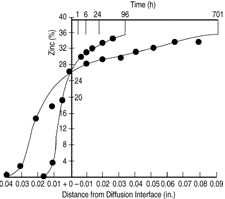
-brass phase, neither a distinct difference in the grain size nor a clear  -brass/copper interface boundary was observed because the crystal structure was the same. Figure 6 depicts the zinc concentration profiles after the different anneals at 1,053 K.
-brass/copper interface boundary was observed because the crystal structure was the same. Figure 6 depicts the zinc concentration profiles after the different anneals at 1,053 K.
 |
| Figure 5. Metallographic observation at the successive diffusion anneal at 1,053 K until 2,523.6 ks: (a) one hour, (b) six hours, (c) 24 hours, (d) 96 hours, (e) 701 hours, and (f) 701 hours. |
where D is diffusivity. Thus, one obtains
 t
tApplying the above equation to the result shown in Figure 6, the average of the diffusivity at 26% Zinc concentration at 1,053 K was
 3.8 X 10-13 m2s-1
3.8 X 10-13 m2s-1
This paper concluded that, at most, one-fifth of the movement of the original interface was due to volume shrinkage accompanied by the phase change from  - to
- to  -brass, while the remaining four-fifth was attributed to the zinc diffusion being faster than that of copper. This explanation was in contrast to that in the first paper2 and in the dissertation.
-brass, while the remaining four-fifth was attributed to the zinc diffusion being faster than that of copper. This explanation was in contrast to that in the first paper2 and in the dissertation.
|
| Figure 6. Zinc concentration profiles after different anneals at 1,053 K. |
There were two main features of the third paper. First, 70-30 brass (70% copper and 30% zinc) was adopted in order to avoid the large volume change from 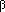 -brass to
-brass to  -brass. Second, insoluble thin wires of molybdenum were inserted in the bonding interface between the copper and the brass for clear observation of the movement of the original interface.
-brass. Second, insoluble thin wires of molybdenum were inserted in the bonding interface between the copper and the brass for clear observation of the movement of the original interface.
|
| Figure 7. A sketch of a cross section of a bar-shaped specimen from the third paper.4 |
 -brass core, molybdenum wires, and a heavy layer of electroplated copper. Figure 7 is a sketch of the cross section of the bar. Diffusion was carried out at 1,058 K for various lengths of time. The distance between the two sets of molybdenum wire were measured, which identified parallel diffusion planes or interfaces. X-ray diffraction was also carried out to obtain diffusion penetration profiles.
-brass core, molybdenum wires, and a heavy layer of electroplated copper. Figure 7 is a sketch of the cross section of the bar. Diffusion was carried out at 1,058 K for various lengths of time. The distance between the two sets of molybdenum wire were measured, which identified parallel diffusion planes or interfaces. X-ray diffraction was also carried out to obtain diffusion penetration profiles.
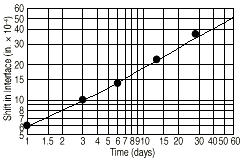
Kirkendall found a shrinkage of the brass core. Figure 8 shows the shift of each interfacehalf the total decrease in interface-to-interface distance. On the basis of the sample analysis described in the second paper, the interdiffusion coefficient was evaluated from these diffusion penetration profiles as
 4 X 10-13 m2 s-1
4 X 10-13 m2 s-1This was in good agreement with the previous value 3.8 X 10-13 m2s-1. In this way, he confirmed good reproducibility. Furthermore, he also confirmed from the metallographic observation that zinc diffused into the copper faster than the copper diffused into the brass.
|
| Figure 8. The annealing time dependence of interface shift. |
 -brass, the zinc diffuses much more rapidly than copper. Such diffusion was accompanied by the shrinkage of the high-zinc
-brass, the zinc diffuses much more rapidly than copper. Such diffusion was accompanied by the shrinkage of the high-zinc  -brass from which zinc diffused out. It was stressed that studies of diffusion and related phenomena consider an unequal interchange of solute and solvent atoms during diffusion and that a mass shift of metal including the interface might result. Finally, he concluded that "diffusion formulas based on an equal interchange of solute and solvent atoms and a substantially stationery interface will be in error."
-brass from which zinc diffused out. It was stressed that studies of diffusion and related phenomena consider an unequal interchange of solute and solvent atoms during diffusion and that a mass shift of metal including the interface might result. Finally, he concluded that "diffusion formulas based on an equal interchange of solute and solvent atoms and a substantially stationery interface will be in error."
Mehl still doubted the Kirkendall paper. Meanwhile, L.C.C. daSilva from Brazil joined his laboratory as a graduate student. In order to verify that the Kirkendall Effect was wrong, Mehl let the student carry out systematic experiments for interdiffusion, not only in Cu/ -brass, but also in Cu/Sn
-brass, but also in Cu/Sn  -solid solution, Cu/Al
-solid solution, Cu/Al  -solid solution, and in Cu/Ni, Cu/Au, and Ag/Au diffusion couples. To the contrary of Mehl's expectation, daSilva found that the marker movement was confirmed and undoubtedly associated with the manner in which atoms move during diffusion (i.e., the Kirkendall Effect was reproducible). Mehl continued to believe, however, that diffusion took place by a direct exchange mechanism so that DA= DB.
-solid solution, and in Cu/Ni, Cu/Au, and Ag/Au diffusion couples. To the contrary of Mehl's expectation, daSilva found that the marker movement was confirmed and undoubtedly associated with the manner in which atoms move during diffusion (i.e., the Kirkendall Effect was reproducible). Mehl continued to believe, however, that diffusion took place by a direct exchange mechanism so that DA= DB.
A diffusion seminar was held October 21-27, 1950, during the 32nd ASM National Metal Congress at Chicago, where top-ranking diffusion researchers such as L.S. Darken, C. Wells, J. Bardeen, C. Herring, H.B. Huntington, F. Seitz, R.F. Mehl, D. Turnbull, and J.E. Burke joined together. The details of the seminar were reported in the literature.9 In this conference most of the attendees approved the validity of the Darken equation, which supported DA DBin interdiffusion, the vacancy mechanism proposed by Huntington and Seitz, and the Kirkendall Effect. During the conference, Seitz thoroughly persuaded Mehl, who, as before, had opposed the vacancy mechanism and the Kirkendall Effect. After a few days, he admitted the validity of the Kirkendall Effect. Mehl himself officially announced in the closing remarks that the Kirkendall Effect was acceptable and that, moreover, his student had much data to confirm the reproducibility of the effect for several alloying systems.10

|
| Ernest Kirkendall |
This question was my motivation to investigate the episode relating to Kirkendall. I was invited to his home and had already spent as long as six hours enjoyably talking with him. If I were to ask such a severe question directly, I was afraid that I would be impolite. But if I did not, I might regret it later without having solved the problem. Accidentally (and fortunately), he offered me his favorite Danish schnapps, Cherry Heering. My feelings became more comfortable, and I realized this was a good opportunity to ask such severe questions. The doctor replied, "My promotion to associate professor had already been approved. The rumor was never reliable! The salary of the secretary of the AIME headquarters in New York City was attractive; it was more than twice the university's just after World War II. I had three children and had to pay high tuitions for their schools and living expenses if living away from home. For this economic reason, I preferred the job change."
I asked him if he would have continued his research career if more people had accepted the Kirkendall Effect earlier, but he replied that he did not attribute much significance to the discovery, at least at that time. Although C.S. Smith tried to persuade him to stay at the university, he preferred the administrative job from an economic point of view, and even if he had accepted the promotion and stayed at the university, he would not have had any positive prospects for his research because of insufficient research facilities.
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|