|
|
|
 |
http://www.tms.org/pubs/journals/JOM/0010/Kumar/Kumar-0010.html
|
|
|
|
 |
http://www.tms.org/pubs/journals/JOM/0010/Kumar/Kumar-0010.html
|
 |
|---|
|
TABLE OF CONTENTS |
|
|
 |
|
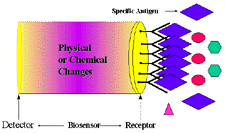
Figure 1. Schematic diagram of a biosensor device. |
Biosensors are chemical sensors that take advantage of the high selectivity and sensitivity of a biologically active material. It is well known that the resonant frequency of an oscillating piezoelectric crystal can be affected by a change in mass at the crystal surface. Piezoelectric immunosensors are able to measure a small change in mass. This paper describes the construction of an antibody-based piezoelectric sensor capable of detecting mycobacterial antigen in diluted cultures of attenuated M. tuberculosis.
A biosensor is an analytical tool consisting of biologically
active material used in close conjunction with a device that will convert a
biochemical signal into a quantifiable electrical signal. Biosensors have many
advantages, such as simple and low-cost instrumentation, fast response times,
minimum sample pretreatment, and high sample throughput. Although biosensors
are beginning to move toward field testing and commercialization in the United
States, Europe, and Japan, relatively few have been commercialized. Increased
research in this area demands the development of novel materials, new and better
analytical techniques, and new and improved biosensors.7-11
Some potential applications of biosensors are agricultural, horticultural and
veterinary analysis; pollution, water and microbial contamination analysis;
clinical diagnosis and biomedical applications; fermentation analysis and control;
industrial gases and liquids; mining and toxic gases; explosives and military
arena; and flavors, essences and pheromones.1-6
A biosensor has two components: a receptor and a detector. The receptor is responsible
for the selectivity of the sensor. Examples include enzymes, antibodies, and
lipid layers. The detector, which plays the role of the transducer, translates
the physical or chemical change by recognizing the analyte and relaying it through
an electrical signal. The detector is not selective. For example, it can be
a pH-electrode, an oxygen electrode or a piezoelectric crystal. Figure
1 describes a typical biosensor configuration that allows measurement of
the target analyte without using reagents. The device incorporates a biological-sensing
element with a traditional transducer. The biological-sensing element selectively
recognizes a particular biological molecule through a reaction, specific adsorption,
or other physical or chemical process, and the transducer converts the result
of this recognition into a usable signal, which can be quantified. Common transduction
systems are optical, electro-optical, or electrochemical; this variety offers
many opportunities to tailor biosensors for specific applications.1-6
For example, the glucose concentration in a blood sample can be measured directly
by a biosensor (which is made specifically for glucose measurement) by simply
dipping the sensor into the sample.
The objective of the research described here is to use biosensor technology
to develop a rapid method for the diagnosis of tuberculosis and other infections
caused by mycobacteria. The work encompassed here describes the construction
of antibody-based piezoelectric crystals capable of detecting mycobacterial
antigens in diluted cultures of attenuated M. tuberculosis in an immunologically
specific manner. The antigen were detected in either liquid or vapor phase.
|
Analytes
|
Examples
|
|
|
|
|
Respiratory Gases
|
O2,
CO2
|
|
Anesthetic Gases
|
N2O,
Halothane
|
|
Toxic Gases
|
H2S,
Cl2, CO, NH3
|
|
Flammable Gases
|
CH4
|
|
Ions
|
H+,
Li+, K+,
Na+, Ca+,
Phosphates, Heavy Metal Ions
|
|
Metabolites
|
Glucose, Urea
|
|
Trace Metabolites
|
Hormones, Steroids, Drugs
|
|
Toxic Vapors
|
Benzene, Toluene
|
|
Proteins and Nucleic Acids
|
DNA, RNA
|
|
Antigens and Antibodies
|
Human Ig, Anti-human Ig
|
|
Microorganisms
|
Viruses, Bacteria, Parasites
|
|
|
|
Two classes of bio-recognition processes-bio-affinity recognition-and bio-metabolic recognition, offer different methods of detection. Both processes involve the binding of a chemical species with another, which has a complementary structure. This is referred to as shape-specific binding. In bio-affinity recognition, the binding is very strong, and the transducer detects the presence of the bound receptor-analyte pair. The most common types of processes are receptor-ligand and antibody-antigen binding. In bio-metabolic recognition, the analyte and other co-reactants are chemically altered to form the product molecules. The biomaterials that can be recognized by the bio-recognition elements are as varied as the different reactions that occur in biological systems. Table I lists a number of common analytes that could prove attractive for developing biosensors of appropriate specificity and sensitivity. Almost all types of biological reactions, (chemical or affinity), can be exploited for biosensors. The concept of shape-specific recognition is commonly used to explain the high sensitivity and selectivity of biological molecules, especially antigen-antibody systems. The analyte molecule has a complementary structure to the antibody, and the bound pair is in a lower energy state than the two separate molecules. This binding is very difficult to break. Table II summarizes a variety of biosystem-transducer combinations in terms of transducer, measurement mode and potential application.
|
Transducer System
|
Measurement Mode
|
Typical Applications
|
|
|
|
|
|
Ion-Selective Electrode
|
Potentiometric
|
Ions in biological media, enzyme electrodes
|
|
Gas-Sensing Electrodes
|
Potentiometric
|
Gases, enzyme, organelle, cell or tissue
electrodes
|
|
Field-Effect Transistors
|
Potentiometric
|
Ions, gases, enzyme substrates immunological
analytes
|
|
Optoelectronic and Fiber-Optic Devices
|
Optical
|
pH; enzymes; immunological analytes
|
|
Thermistors
|
Calorimetric
|
Enzyme, organelle, gases, pollutants,
antibiotics, vitamins
|
|
Enzyme Electrodes
|
Amperometric
|
Enzymes, immunological systems
|
|
Conductimeter
|
Conductance
|
Enzyme substrates
|
|
Piezoelectric Crystals
|
Acoustic (mass)
|
Volatile gases and vapors, antibodies
|
|
|
||
The interaction of antibodies with their corresponding antigens
is an attractive reason for attempting to develop antibody-based chemical biosensors,
i.e. immunosensors. Theoretically, if an antibody can be raised against a particular
analyte, an immunosensor could be developed to recognize it. Despite the high
specificity and affinity of antibodies towards complementary ligand molecules,
most antibody-antigen interactions do not cause an electronically measurable
change. However, the remarkable selectivity of antibodies has fueled much research
to overcome this intrinsic problem. The piezoelectric effect in various crystalline
substances is a useful property that leads to the detection of analytes. Figure
2 shows a schematic diagram of an immunosensor device.12-21
|
|
|
Figure 2. Schematic diagram of an immunosensor device. |
|
|
The piezoelectric immunosensor is thought to be one of the
most sensitive analytical instruments developed to date, being capable of detecting
antigens in the picogram range. Moreover, this type of device is believed to
have the potential to detect antigens in the gas phase as well as in the liquid
phase.
Almost all current methods of diagnosing tuberculosis (TB) have drawbacks. They
tend to be either nonspecific or too time-consuming. In most cases of pulmonary
and extrapulmonary TB, diagnosis depends upon culturing the mycobacterial organism,
a process requiring 4-8 weeks. Significant attention has been devoted to developing
more rapid diagnostic methods for TB, but some of them do not have the high
specificity or sensitivity required for proper diagnosis.22-29
A piezoelectric sensor that could reliably detect the mycobacterial antigen
in biological fluids would be of enormous use. For instance, detection of the
antigen in saliva could constitute a noninvasive method of screening high-risk
populations. One tested piezoelectric crystal sensor gives results within a
couple of hours after exposing the electrode to a liquid containing the antigen.
The apparatus would be quite portable, so the immunological tests could be performed
virtually anywhere, and the results could be obtained very quickly. The feasibility
of using piezoelectric immunosensors to diagnose TB based upon the detection
of mycobacterial antigens in liquid depends upon the degree of sensitivity and
specificity that can be achieved and upon overcoming any problems caused by
potentially interfering substances in biological fluids. The feasibility of
gas- or vapor-phase detection of antigen depends upon these same factors, plus
any difficulties that may be unique to gas-phase antigen capture by antibodies.
Theoretical Principals
The basic equations describing the relationship between the resonant frequency
of an oscillating piezoelectric crystal and the mass deposited on the crystal
surface have been derived by Sauerbrey,30
Stockridge,31 and Lostis.32
Each followed a different path, but their final equations are similar, the Sauerbrey
equation being the most widely accepted. In 1959, Sauerbrey developed an empirical
equation for AT-cut quartz crystals vibrating in the thickness shear mode that
describes the relationship between the mass of thin metal films deposited on
quartz crystals and the corresponding change in resonant frequency of the crystal:
|
|
(1)
|
|
|
(2)
|
|
|
(3)
|
|
|
(4)
|
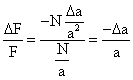 |
(5)
|
|
|
(6)
|
|
|
(7)
|
|
|
(8)
|
|
|
(9)
|
|
|
(10)
|
|
DF = -k DM
|
(11)
|
|
|
(12)
|
|
DF |
|
|
|
(13)
|
|
V = q · t
|
(14)
|
|
|
(15)
|
|
|
(16)
|
|
|
(17)
|
|
DF = K C
|
(18)
|
|
|
|
Figure 3. Quartz crystal and holder. |
|
|
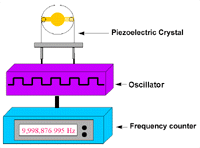
Figure 4. Experimental apparatus for a piezoelectric sensor. |
 |
|
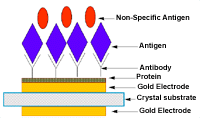
Figure 5. Schematic diagram of the antibody-antigen binding. |
Electrode Fabrication Process
The most frequently used detector crystal is alpha quartz. These crystals are
most suitable for piezoelectric application because they are insoluble in water
and resistant to high temperatures. Alpha quartz crystals can be resistant to
temperatures up to 579°C with no loss of piezoelectric properties. The resonant
frequency of quartz crystal depends on the physical dimensions of the quartz
plate and the thickness of the electrode deposited. AT and BT-cut crystals are
most useful as piezoelectric detectors. These cuts refer to the orientation
of the plate with respect to the crystal structure. The AT-cut crystal is the
most stable, with a temperature coefficient of 1 ppm per degree centigrade over
a temperature range of 10°C to 50°C. The crystals usually take the form of discs,
squares, and rectangles.
All crystals in this investigation were general-purpose 10 MHz AT-cut quartz
crystals with an electrode coating deposited on each side using sputtering method.
The crystal was mounted on a holder with stainless steel with leads. A silver
composite was used to connect the electrode to wire. The crystals were 14 mm
in diameter, and the electrodes on both sides of the crystal were 8 mm in diameter.
The crystals were mounted on size HC6/u holders. Figure
3 shows the schematic diagram of the fabricated crystal attached to the
base.
Figure 4 is a block diagram of the apparatus
used for the biosensor experiment. The piezoelectric quartz crystal was driven
by a low-frequency transistor oscillator, powered by a 1-30 V d.c. regulator
power supply and set at 9 V d.c. The frequency of the vibrating crystal was
monitored by a Protek multifunction frequency counter. The crystal mounted on
its holder was connected to the oscillator circuit and the frequency counter
was connected to the oscillator device. After each step in the coating process-first
with the various metal depositions and then with the biomolecular analytes-the
frequency reading was recorded.33
Immunosensing
The crystal electrodes were first modified with a 5 ml coating of protein A
for better adhesion of the antibodies to the surface of the transducer. Protein
A is a polypeptide isolated from Staphylococcus aureus that binds specifically
to the immunoglobulin molecules, especially IgG antibodies, without interacting
at the antigen site. This property permits the formation of tertiary complexes
consisting of protein A, antibody, and antigen. Prior to modification, the electrodes
were anodically oxidized at constant current in 0.5 M NaOH. They were then cleaned
in 0.5 M HCl and 0.5 M HCrO2.
They were dried in an incubator for one hour, and the antibody (IgG) coating
was then applied to the protein A coating. Using a pipette, 10 ml of antibody
was applied on both sides of the crystal. After another hour of drying, 10 ml
of antigens were coated onto the crystal, and they were methodically dried again.
After each step, the frequency of the crystal was recorded, and the crystal
was washed as a precaution against non-specific binding.
Control crystals and experimental crystals were coated with antibodies. The
former were coated with an irrelevant antibody (HBV-honey bee antibody), one
specific for an antigen not present in the solution containing the analyte.
The experimental sample was coated with antibody (M. tuberculosis) specific
for binding to the antigen. Both were exposed to the solution containing the
analyte. Then the difference in frequency change between the control and experimental
crystals were compared, reflecting the immunologically specific binding of analyte.
This procedure was carried out first with crystal with gold substrate, and then
using crystals coated with magnetic materials. A magnetic field was induced
during the investigation of the latter. The nature of the binding of antigen
to antibody to the surface of the transducer is shown in Figure
5. The protein helps the antibody to bind to the electrode and the antigen
in gaseous state binds to specific antibody.33
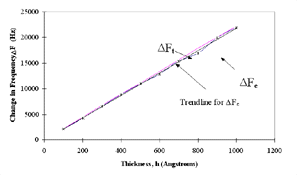
Figure 6 shows the x-ray diffraction patterns of the gold films deposited on quartz. The gold film is polycrystalline in nature. The theoretical values at varying gold thicknesses, compared with experimental values at different thicknesses, are shown in Figure 7, which clearly shows that the experimental and theoretical values of change in frequency are almost equal for different thicknesses of electrode coating. The deposited coating changes the frequency approximately in agreement with the projections made by Sauerbrey.
|
|
 |
|
|
Figure 6. X-ray diffraction pattern of gold electrode coating on a quartz substrate. |
Figure 7. Frequency change (experimental and theoretical) vs. thickness for the deposited gold coating. |
|
|
|
|
Although platinum is a more noble (non-reactive) material compared to the
gold, the adherence of the platinum films was very poor on quartz substrates
and the platinum reacted with different buffer solutions, (e.g. HCl and NaOH)
during the specimen preparation for antigen-antibody binding. Thus, gold electrodes
are preferred due to superior adhesion and non-reactive properties. The optimum
thickness of the gold electrode layer was estimated at 1,000 Angstroms. Although
the binding between antigen and antibody did show a change in frequency, the
results were not always reproducible. The antibody binding to the protein layer
was critical to achieve desirable results. The use of magnetic materials underneath
the gold coating helped make the antigen detectable.33-35
These results are preliminary. More analysis is needed to shed more light on the optimum binding characteristics of the antigen and antibody to the piezoelectric transducer.
This research was supported by a National Science Foundation grant. The author thanks S. Perlaky, I. Hussain, and A. Mangiaracina for their contributions in doing research at the University of South Alabama.
1. A.S. Dewa
and W.H. Ko, "Biosensors," Semiconductor Sensors, ed. S.M. Sze (New York:
Wiley Interscience,
1994), p. 415.
2. Elizabeth A.H. Hall, ed.,
Biosensors (New York: Prentice
Hall, 1991).
3. D. Diamond, ed., Principles
of Chemical and Biological Sensors, vol. 150 (New York: John
Wiley & Sons, 1998).
4. C.R. Lowe, "An Introduction
to the Concepts and Technology of Biosensors," Biosensors,
1 (1985), p. 4.
5. K.R. Rogers, "Biosensors
for Environmental Applications," Biosensors
Bioelectronics, 10 (1995), pp. 533-541.
6. A.P.F. Turner, "Current Trends
in Biosensor Research and Development," Sensors Actuators, 17 (1989),
pp. 433-450.
7. E.C. Hahn, "Piezoelectric
Crystal Detectors and Their Applications," Ph.D. dissertation, University of
New Orleans, 1988.
8. W.P. Mason, Piezoelectric
Crystals and Their Application to Ultrasonics (Princeton, NJ: Van Nostrand,
1950).
9. G.G. Guilbault and J.M. Jordan,
"Analytical Uses of Piezoelectric Crystals: A Review," CRC
Crit. Rev. Anal. Chem., 19 (1) (1988), pp. 1-28.
10. R.A. Heising, Quartz
Crystal for Electrical Circuits (New York: Van Nostrand, 1946), p. 24.
11. M. Ho, Applications
of Piezoelectric Quartz Crystal Microbalances, ed. C. Lu and A.W. Czanderna
(Amsterdam: Elsevier/North
Holland, 1984).
12. J.H.T. Luong and G.G. Guilbault,
"Analytical Applications of Piezoelectric Crystal Biosensors," Biosensor
Principles and Applications, ed. L.J. Blum and P.R. Coulet (New York: Marcel
Dekker, 1991), pp. 107-138.
13. G.G. Guilbault, "Detection
of Formaldehyde with an Enzyme-Coated Piezoelectric Crystal Detector," Anal.
Chem., 55 (1983), pp. 1682-1684.
14. J. Ngeh-Ngwainbi et al.,
"Parathion Antibodies on Piezoelectric Crystals," J.
Am. Chem. Soc., 108 (1986), pp. 5444-5447.
15. G.G. Guilbault, B. Hock,
and R. Schmid, "A Piezoelectric Immunobiosensor for Atrazine in Drinking Water,"
Biosensors
Bioelectronics, 7 (1992), pp. 411-420.
16. M. Minunni, P. Skladal,
and M. Mascini, "A Piezoelectric Quartz Crystal Biosensor for Atrazine," Life
Chemistry Reports, 11 (1994), p. 391.
17. M. Minunni, P. Skladal,
and M. Mascini, "A Piezoelectric Quartz Crystal Biosensor as a Direct Affinity
Sensor," Anal. Lett., 27 (1994), p. 1475.
18. K. Nakanishi et al., "Detection
of the Red Tide-Causing Plankton Alexandrium Affine by a Piezoelectric Immunosensor
Using a Novel Method of Immobilizing Antibodies," Anal. Lett., 29 (1996),
pp. 1247-1258.
19. M. Minunni, P. Skladal,
and M. Mascini, "A Piezoelectric Quartz Crystal Biosensor as a Direct Affinity
Sensor," Anal. Lett., 27 (1994), pp. 1475-1487.
20. K.R. Rogers and E.N. Koglin,
"Biosensors for Environmental Monitoring: An EPA Perspective," Biosensors
for Direct Monitoring of Environmental Pollutants in Field, ed. D.P. Nikolelis
et al. (Norwell, MA: Kluwer Academic
Publishers, 1997), pp. 335-349.
21. H. Muramatsu et al., "Piezoelectric
Crystal Biosensor Modified with Protein A for Determination of Immunoglobulins,"
Anal. Chem., 59 (1987), pp. 2760-2763.
22. C.J.L. Murray, K. Styblo,
and A. Rouillon, Disease Control Priorities in Developing Countries,
ed. D.T. Jamison and W.H. Mosley (New York: Oxford
University Press, 1990), p. 50.
23. B.R. Broom and C.J.L. Murray,
"Tuberculosis: Commentary on a Reemergent Killer," Science,
257 (1992), pp. 1055-1064.
24. M.F. Goldsmith, "Medical
Exorcism Required as Revitalized Revenant of Tuberculosis Haunts and Harries
the Land," JAMA,
268 (2) (1992), pp. 174-175.
25. P.F. Barnes et al., "Tuberculosis
in Patients with Human Immunodeficiency Virus Infection," N.
Engl. J. Med., 324 (1991), pp. 1644-1650.
26. A.D. Harries, "Tuberculosis
and Human Immunodeficiency Virus Infection in Developing Countries," Lancet,
335 (1990), pp. 387-390.
27. J.C. Weissler, "Southwestern
Internal Medicine Conference: Tuberculosis-Immunopathogenesis and Therapy,"
Amer. J. Med. Sci., 305 (1993), pp. 52-65.
28. D.E. Sada, L.E. Ferguson,
and T.M. Daniel, "An ELISA for the Serodiagnosis of Tuberculodid Using a 30,000-Da
Native Antigen of Mycobacterium Tuberculosis," J.
Infect. Dis., 162 (1990), pp. 928-931.
29. T.C. Huang et al., "Analysis
of Cobalt Doped Iron Oxide Thin Films by Synchrotron Radiation," Thin
Solid Films, 154 (1987), pp. 439-445.
30. G. Sauerbrey, Z. Phys.,
155 (1959), p. 206.
31. C.D. Stockbridge, Vac.
Microbalance Tech., 5 (1996), p. 193.
32. M. Lostis, Ph.D. dissertation,
Faculty of Science, University of Paris, 1958.
33. I. Hussain, "Development
and Applications of Piezoelectric Biosensors," M.S. thesis, University
of South Alabama, 1998.
34. I. Hussain et al., "Fabrication
of Piezoelectric Sensors for Biomedical Applications," MRS Symp. Proc. Materials
for Smart System, 459 (1997), pp. 501-506.
35. Ashok Kumar et al., "Design
and Implementation of a Piezoelectric Biosensors for Multifunctional Applications,"
Integrated Design and Process Technology, IDPT-Vol . 1 (1998), pp. 35-41.
Ashok Kumar is with the Department of Mechanical Engineering and Center for Microelectronic Research at the University of South Florida.
For more information, contact Ashok Kumar, University of South
Florida, Department of Mechanical Engineering and Center for Microelectronics
Research, Tampa, Florida 33620.
Direct questions about this or any other JOM page to jom@tms.org.
| If you would like to comment on the October
2000 issue of JOM,
simply complete the JOM on-line critique form |
|||||
|---|---|---|---|---|---|
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |