 |
52 (12) (2000), pp. 22-27 |
|---|
Solidification Science: Overview
 |
52 (12) (2000), pp. 22-27 |
|---|
|
TABLE OF CONTENTS |
|---|
|
|
The study of metallic foams has become attractive to researchers interested in both scientific and industrial applications. In this paper, various methods for making such foams are presented and discussed. Some techniques start from specially prepared molten metals with adjusted viscosities. Such melts can be foamed by injecting gases or by adding gas-releasing blowing agents which cause the formation of bubbles during their in-situ decomposition. Another method is to prepare supersaturated metal-gas systems under high pressure and initiate bubble formation by pressure and temperature control. Finally, metallic foams can be made by mixing metal powders with a blowing agent, compacting the mix, and then foaming the compact by melting. The various foaming processes, the foam-stabilizing mechanisms, and some known problems with the various methods are addressed in this article. In addition, some possible applications for metallic foams are presented.
Solid metallic foams are known for their interesting combinations
of physical and mechanical properties such as high stiffness in conjunction
with very low specific weight or high compression strengths combined with good
energy absorption characteristics. Although interest in these materials is
increasing, some confusion exists concerning the term “metallic foam,” which
is often used in a general way to describe materials that are not foams in the
strictest sense.
To properly identify a metallic foam, one has to distinguish between
This paper will focus on foams in the strictest sense—liquid-gas
mixtures, in the first stage of their evolution, which are solidified to solid
foam. As surface tension creates a morphology in the liquid state (isolated
gas bubbles which are separated from each other by metal films) the corresponding
solid-metal foams show a similar morphology.
The manufacture of cellular metals in the most general sense, as described in
published works,1 does
not always involve foaming methods. Often, a polymer foam is first opened by
a special treatment and then replicated to yield a metallic structure. Replication
can be carried out by coating with metal vapor, electroplating, or investment
casting. The result is a structure with open porosity— a sponge rather than
a foam. The physics of foaming has nothing to do with the metallic state because
only the polymer precursor was foamed. Other structures can be used as templates
for creating cellular materials: loose or sintered bulks of inorganic or organic
granular matter, hollow spheres, or even regular polymer structures which are
converted to a metallic structure in a designated processing step. In contrast,
true foaming methods do not use a template for obtaining the special morphology;
the metal is self-forming during foaming.
|
|
 |
|
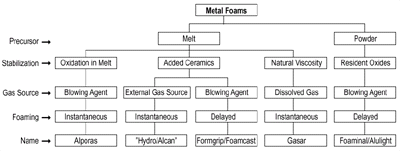
Figure 1. A family tree of metal foams. |
Figure 1 provides an
overview of the methods available for making metal foams.1
One distinguishing factor is whether molten metal or metal powder is used (although
the actual foaming always takes place in the liquid state). A second difference
is the gas source used for creating porosity: an external source can be used,
a blowing agent can be decomposed in-situ, or dissolved gas can be forced to
precipitate. Third, foaming can be instantaneous (i.e., addition of gas leads
to immediate foaming), or an intermediate product is created that can be foamed
in a later stage (delayed foaming). Finally, the mechanism of foam stabilization
is different for the various methods as will be explained later. Some methods
have been given a name, others were given a commercial name by the manufacturer.
|
|
 |
|
|
 |
|
|
 |
|
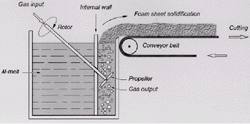
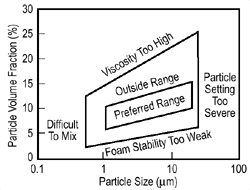
Figure 2. (a-top) Direct foaming of melts by gas injection;2 (b-center) preferable range of stabilizing powders;4 and (c-bottom) a sample made by Hydro-Aluminium. |
Under certain circumstances metallic melts can be foamed by
creating gas bubbles in the liquid. Normally, gas bubbles formed in a metallic
melt tend to quickly rise to its surface due to the high buoyancy forces in
the high-density liquid. This rise can be hampered by increasing the viscosity
of the molten metal, either by adding fine ceramic powders or alloying elements
to form stabilizing particles in the melt or by other means. Metallic melts
can be foamed in one of three ways: by injecting gas into the liquid metal from
an external source, by causing an in-situ gas formation in the liquid by admixing
gas-releasing blowing agents to the molten metal, or by causing the precipitation
of gas which was previously dissolved in the liquid.
Foaming of Melts by Gas Injection (Hydro/Alcan)
The first method of foaming aluminum and aluminum alloys is being exploited
by Hydro Aluminium in Norway
and by Cymat Aluminium Corporation
in Canada.2,3
According to this process, described schematically in Figure2a,
silicon-carbide, aluminum-oxide, or magnesium-oxide particles are used to enhance
the viscosity of the melt. Therefore, the first step comprises the preparation
of an aluminum melt containing one of these substances, making it a metal-matrix
composite (MMC). This step reportedly requires sophisticated mixing techniques
to ensure a uniform distribution of particles. A variety of aluminum alloys
can be used.
The melt is foamed in a second step by injecting gases (air, nitrogen, argon)
into it using specially designed rotating impellers or vibrating nozzles. These
generate very fine gas bubbles in the melt and distribute them uniformly. The
resultant viscous mixture of bubbles and metal melt floats up to the surface
of the liquid where it turns into a fairly dry liquid foam as the liquid metal
drains out. Because ceramic particles are in the melt, the foam is relatively
stable. It can be pulled off the liquid surface (e.g. with a conveyor belt)
and is then allowed to cool down and solidify. The resulting solid foam is,
in principle, as long as desired, as wide as the vessel containing the liquid
metal allows it, and typically 10 cm thick. The volume fraction of the reinforcing
particles typically ranges from 10% to 20% with a mean particle size from 5
mm to 20 mm. The choice
of particle size and content has been carried out empirically. If content or
particle sizes are too high or too low problems can result, as shown in Figure
2b. The densities of aluminum foams produced this way range from 0.069 g/cm3
to 0.54g/cm3 ,average pore sizes from 25 mm
down to 3mm, and wall thicknesses from 50 mm to 85
mm.5
The average cell size is inversely related both to the average cell wall thickness
and to the density and can be influenced by adjusting the gas flow, the impeller
speed, nozzle vibration frequency, and other parameters.
A natural consequence of gravitationally induced drainage6
is evident in foamed slabs (Figure 2c),
which usually have a gradient in density, pore size, and pore elongation. Moreover,
the shearing forces of the conveyor belt lead to diagonally distorted cells
in the final product, causing a pronounced effect on the mechanical properties,
which become an isotropic.7
To avoid such results, the foam can be pulled off vertically. The foamed material
is either used with a closed outer surface (its state upon coming out of the
casting machine) or is cut into the required shape after foaming. The high content
of ceramic particles can make machining of MMC foams difficult.
Advantages of the direct-foaming process include the capability for continuous
production of a large volume of foam and the low densities that can be achieved.
MMC foams are, therefore, probably less expensive than other cellular metallic
materials. A possible disadvantage of the direct-foaming process is the eventual
necessity for cutting the foam, thereby opening the cells.
Foaming pure, additive-free metallic melts with inert gases may be a means to
avoid some of the unwanted side effects of stabilizing additives in metallic
melts (e.g., brittleness).1
To keep viscosity low, the foaming process has to take place at temperatures
very close to the melting point. This can be done by bubbling gas through a
melt which is constantly cooled down (e.g., in a continuous casting process).
The bubbles are then caught in the solidifying liquid and form a foam-like structure.
In the liquid state such systems are very unstable compared to particle-stabilized
metals, which can be kept liquid for some time.
Foaming of Melts with Blowing Agents (Alporas)
|
|
|
 |
|
|
|
|
|
 |
|
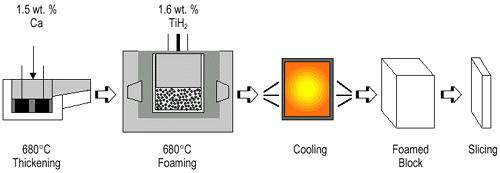
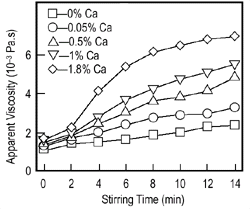
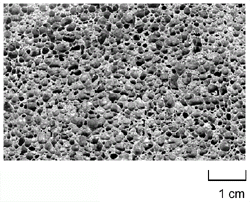
Figure 3. (a-top) Direct foaming of melts by adding gas-releasing powders (Alporas-process);8 (b-bottom left) viscosity vs. stirring time;9 and (c-bottom right) the pore structure of foam (Southeast University, China). |
|
A second way for foaming melts directly is to add a blowing
agent to the melt instead of injecting gas into it. Heat causes the blowing
agent to decompose and release gas, which then propels the foaming process (Figure
3a).8 Shinko Wire
Company, Amagasaki, Japan, has been producing foams in this way since 1986 with
production volumes reportedly up to 1,000 kg per day. In a first step, about
1.5 wt.% calcium metal is added to an aluminum melt at 680°C. The melt is stirred
for several minutes, during which its viscosity continuously increases by a
factor of up to five because of the formation of calcium oxide (CaO), calcium-aluminum
oxide (CaAl2O4),
or perhaps even Al4Ca intermetallics, which
thicken the liquid metal. Figure 3b
shows the effect of stirring on the viscosity of aluminum melts with various
calcium additions.9 After
the viscosity has reached the desired value, titanium hydride (TiH2)
is added (typically 1.6 wt.%), serving as a blowing agent by releasing hydrogen
gas in the hot viscous liquid. The melt soon starts to expand slowly and gradually
fills the foaming vessel. The foaming takes place at constant pressure. After
cooling the vessel below the melting point of the alloy, the liquid foam turns
into solid aluminum foam and can be taken out of the mold for further processing.
The entire foaming process can last 15 minutes for a typical batch (2,050 mm
´ 650 mm ´ 450 mm3).
A careful adjustment of process parameters has been shown to lead to homogeneous
foams (Figure 3c). In fact, the foams
produced in this way—trade name Alporas—seem to be the most homogeneous aluminum
foams currently available. An empirical relationship exists not only between
average cell diameter and the viscosity of the melt but also between the final
foam density and viscosity.9
Typical densities after cutting off the sides of the cast foam blocks are between
0.18 g/cm3 and 0.24 g/cm,3
with the average pore size ranging from 2 mm to 10 mm. The viscosity of molten
aluminum can also be enhanced by bubbling oxygen, air, or other gas mixtures
through the melt, thus causing the formation of alumina; by adding powdered
alumina, aluminum dross, or scrap foamed aluminum; or by using metallic viscosity-enhancing
additives. However, the proper adjustment seems to be quite difficult and requires
complicated temperature cycles and mechanical agitation.
Solid-Gas Eutectic Solidification (Gasar)
A method developed about a decade ago10
exploits the fact that some liquid metals form a eutectic system with hydrogen
gas. If one of these metals is melted in a hydrogen atmosphere under high pressure
(up to 50 atms), the result is a homogeneous melt charged with hydrogen. If
the temperature is lowered, the melt will eventually undergo a eutectic transition
to a heterogeneous two-phase system (solid + gas). If the composition of the
system is sufficiently close to the eutectic concentration, a segregation reaction
will occur at one temperature. As the melt is solidified, gas pores precipitate
and are entrapped in the metal. The resulting pore morphologies are largely
determined by the hydrogen content, the pressure over the melt, by the direction
and rate of heat removal, and by the chemical composition of the melt. Generally,
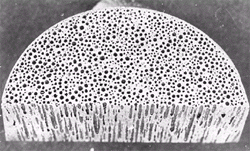
largely elongated pores oriented in the direction of solidification are formed
(Figure 4). Pore diameters range from
10 mm to 10 mm, pore lengths from 100 mm
to 300 mm, and porosities from 5% to 75%. The pore size distribution is non-uniform
because of concurrent growth of small and large pores and coalescence. Pores
may be conical or even corrugated. The word “gasar” was coined to refer to the
porous materials formed by solid-gas eutectic solidification. Gasar is a Russian
acronym meaning “gas-reinforced.”
|
|
 |
|
Figure 4. Gasar, showing largely elongated pores (V. Shapovalov). |
 |
|
|
 |
|
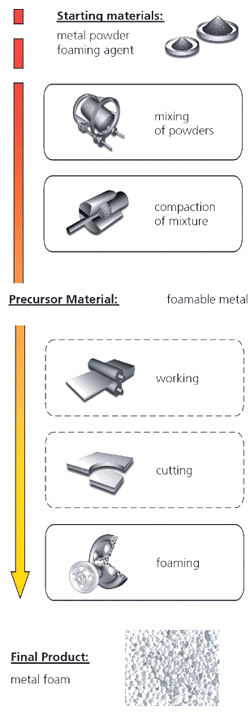
Figure 5. (a-top) The principle of powder-compact foaming method,11 (b-bottom) steel/aluminum foam/ steel sandwich (Fraunhofer, Bremen). |
 |
|
Figure 6. Two samples of aluminum/silicon-carbide foam.19 |
Foaming of Powder Compacts (Foaminal/Alulight)
Foamed metals can be also be prepared from metal powders.11,12
The production process begins with the mixing of metal powders—elementary metal
powders, alloy powders, or metal powder blends—with a blowing agent, after which
the mix is compacted to yield a dense, semi-finished product (Figure
5a). The compaction can be achieved using any technique in which the blowing
agent is embedded into the metal matrix without any notable residual open porosity.
Examples of such compaction methods are uniaxial or isostatic compression, rod
extrusion, or powder rolling. The precursor has to be manufactured very carefully
because residual porosity or other defects will lead to poor results in further
processing. The next step is heat treatment at temperatures near the melting
point of the matrix material. The blowing agent, which is homogeneously distributed
within the dense metallic matrix, decomposes and the released gas forces the
melting precursor material to expand, forming its highly porous structure. The
time needed for full expansion depends on the temperature and size of the precursor
and ranges from a few seconds to several minutes. The method is not restricted
to aluminum and its alloys; tin, zinc, brass, lead, gold, and some other metals
and alloys can also be foamed with appropriate blowing agents and process parameters.
If a piece of precursor material is foamed in a furnace, the
result will be a lump of metal foam with an undefined shape unless the expansion
is limited. This is done by inserting the precursor material into a hollow mold
and expanding it by heating, creating near-net shaped parts with a closed outer
skin and a highly porous cellular core.13
Complicated parts can be manufactured by injecting the still-expanding foam
from a reservoir into suitable molds.14
Sandwich panels consisting of a foamed metal core and two metal face sheets
can be fairly easily obtained by bonding the face sheets to a piece of foam
with adhesives. Alternatively, if pure metallic bonding is required, conventional
sheets of metal—aluminum or steel—are roll-clad to a sheet of foamable precursor
material.15,16
The resulting composite can be deformed in an optional step, e.g., deep drawing.
The final heat treatment, in which only the foamable core expands and the face
sheets remain dense, then leads to sandwich structures such as the one shown
in Figure 5b. Aluminum foam can be combined
with steel or titanium face sheets as well as with aluminum face sheets. In
the latter case, alloys with melting points that are different from the core
material and the face sheets must be used to avoid melting the face sheets during
foaming. A large aluminum/aluminum foam sandwich was developed in a joint effort
by the German car maker Karmann in Osnabrück and Fraunhofer-Institute in Bremen
for a concept car in which structural aluminum foam applications were demonstrated.17
Such sandwiches are three-dimensional, up to two meters long and about one meter
wide.
The powder-compact melting method is in small-scale commercial by the German
companies Schunk (Giessen) and Honsel (Meschede) and the Austrian companies
Alulight (Ranshofen)
and Neuman Alufoam (Marktl). The names “Foam-in-Al” and “Alulight” have been
coined for these foams.
Foaming of Ingots Containing Blowing Agents (Formgrip/Foamcast)
The powder-compact melting process was recently modified by incorporating titanium-hydride
particles directly into an aluminum melt instead of using powders to prepare
a foamable precursor material. To avoid premature hydrogen evolution the melt
has to be either quickly cooled down below its melting point after mixing or
the blowing agent has to be passivated to prevent it from releasing gas before
solidification. The former technique, named “Foamcast,” was carried out in a
die-casting machine, when the powdered hydride was injected into the die simultaneously
with the melt.18 Normal
casting alloys such as A356 without ceramic additives were used. The resulting
cast part was virtually dense and could be foamed by remelting in analogy to
the powder-based method described previously. However, achieving a homogeneous
distribution of TiH2 powders in the die is
challenging. The latter route requires that TiH2
powders be subjected to a cycle of heat treatments that form an oxide barrier
on each particle and delay decomposition. The powders are then added to a melt
and can be cooled at comparatively slow rates after stirring.19
Melts containing silicon carbide are used to obtain stable foams. The foaming
process can be influenced by varying heating rates and final foaming temperatures,
thus allowing for producing a variety of different pore structures (Figure
6). The process has been named “Formgrip,” which is an acronym of foaming
of reinforced metals by gas release in precursors.
Foams are unstable systems because their large surface area causes energy to
be far from a minimum value. Foams can therefore be, at the most, metastable,
constantly decaying at a certain rate. With foams, then, stability is the equivalent
of slow decay. Aqueous and non-aqueous foams are stabilized by surfactants which
form a dense mono-layer on a foam film. Such layers reduce surface tension,
increase surface viscosity, and create electrostatic forces (the so-called disjoining
forces) to prevent a foam film from collapsing.20
Metallic foams must be stabilized by different means because there are no surfactants
and electrostatic forces are screened in metals. Like water, pure metallic melts
cannot be foamed, but additives are required to act as stabilizers to create
a foam.
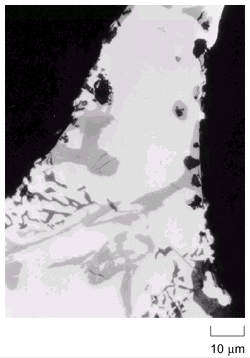
Silicon-carbide particles, for example, were added to the melt in the Hydro/Alcan
foaming process. These particles, typically about 10 mm
in diameter, were proven necessary by measuring foaminess as a function of particle
content. It was found that 8–20 vol.% silicon carbide in aluminum was needed
depending on how the gas is injected into the liquid.21
A micrograph of a foam of this type is shown in Figure
7. The original composition of the material was not communicated, but presumably
it contained about 7 wt.% silicon, some magnesium, and about 15 wt.% silicon
carbide. Inclusions are evident, all with angular contours but with varying
shapes and colors. Although an analysis by energy-dispersive x-ray analysis
(EDX) yielded a variety of different compositions, very small particles are
difficult to analyze because EDX recorded an average over a circular area of
8 mm diameter and 3 mm
depth. However, one can find regions which contain MgO (fairly large with d
» 20 mm), fine Al-Si near-eutectic
regions, iron-rich phases, and, finally, silicon-carbide particles with a dispersion
of size. The inclusions can be found all over the cross section of the foam
films with a slight tendency for an accumulation at the film surfaces.
In the Alporas process, additives are aluminum-, calcium-, or mixed oxides,
which are formed in the melt by internal oxidation after adding calcium metal
and stirring. The source of oxygen could be alumina or other oxides that are
in the melt before adding calcium, or air that is dragged into the melt during
agitation. A micrograph of an Alporas foam (Figure
8) shows two different types of precipitates: light gray precipitates, the
majority of which are about 10 mm in diameter, and
a small fraction of smaller, dark gray inclusions, about 3 mm
in diameter, that are connected to the light gray particles. An EDX analysis
finds the light gray areas contain roughly 5 at.% calcium, 12.5 at.% titanium,
and 5 at.% oxygen, the dark areas 12 at.% calcium, 2 at.% titanium, and 6 at.%
oxygen. The dark areas, because of their small size, could not be measured precisely,
however. The values obtained are in a reasonable agreement with values found
in published reports.6
The precipitates likely contain various mixed oxides of aluminum, calcium, and
titanium such as Al2CaO4
or Al2Ca3O6,
or oxide mixes Al2O3+TiO2,22
or intermetallic compounds such as Al4Ca,
Al2Ca, or Al3Ti.23,24
There is no evidence that the precipitates are concentrated on or near the bubble/air
interface but they seem to be fairly evenly distributed over the cross section
of the cell walls. One can easily find parts of the bubble surface which do
not contain any precipitates. This casts doubts on the hypothesis that solid
particles floating on the walls of films are responsible for their stabilization
in analogy to the action of surfactants in aqueous foam.21,2
In the Foaminal/Alulight process, the stabilization can be ascribed to metal-oxide
filaments which reside in the powder compacts used, because oxides cover the
surface of each powder particle prior to solidification and remain in the compact
after pressing. These filaments are very thin, especially for aluminium where
their thickness is believed to be well below 100 nm. The important role of these
oxides in foam stabilization is shown in Figure
9. Lead foams were manufactured by mixing lead powders with different degrees
of oxidation with a blowing agent, compacting the mix, and foaming it. Powders
with very low oxide contents lead to unstable foams; as the foam rises liquid
drains from it and limits its expansion. More stable foams result when powders
with higher oxide contents are used and a large part of the liquid lead is kept
in the foam structure at least until maximum expansion has been reached. There
is also some evidence that the same mechanisms are effective for aluminum.27
|
|
|
|
||
 |
 |
 |
||
|
Figure 7. Microstructure of foam made by injecting gas into silicon-carbide-reinforced melt (Alcan foam). |
Figure 8. Microstructure of the cell wall material of an Alporas foam. |
Figure 9. Lead foams made from two different lead powders. (a-top) Low-oxygen powder (0.06 wt.%) and (b-bottom) higher oxidized powder containing 0.46 wt.% O.26 |
||
|
|
|
|
Metallic foams, therefore, appear to be stabilized by solid particles. The
action of foam stabilization is not entirely clear yet, but some current ideas
on metal foams have been published 19,21,25
as well as general information on foams.20
There are two questions to be discussed in this context. First, where are the
solid particles located in the foam? Second, are they incorporated into the
metal or do they segregate? Their behavior is governed by the wettability of
the particles by the melt, commonly described by the contact angle between the
two. This angle is determined primarily by the chemical composition of the particle
but probably also by its size, shape, surface roughness, and concentration in
the liquid. The particles in the silicon-carbide stabilized aluminum foams of
the Hydro/Alcan- and Formgrip-type have been said to be partially wetted as
they accumulate on the inner walls of bubbles.19,21
This view, however, is supported neither by the work of the author (Figure
7) nor the micrographs shown in Figure
6. Surface oxides have not yet been directly observed in the foams made
by powder-compact melting. All that is known is that aluminum-powder compacts
have oxide contents up to 1 wt.% and that the foams show oxide layers on their
surfaces that are 30 nm thick after foaming under argon.27
However, part of this oxide might have formed by reactions with residual oxygen
in the foaming chamber. Recent real-time x-ray observations of the aluminum-foaming
process have revealed that oxidation of evolving foams increases the apparent
viscosity of films that are near the surface and, therefore, exposed to oxygen.28
However, this effect should not be confused with the postulated effect of particles
accumulating on the film surface.
Second, how does a given configuration of ceramic particles and metal films
influence foam stability? Various mechanisms have been proposed:
In conclusion, although the stabilization mechanism is still not well understood,
I believe the use of solid particles to enhance the viscosity of a metallic
melt is the main means to stabilize the structure. A surface effect by partially
wetted particles in analogy to the action of surfactants in aqueous foams seems
rather implausible. The opposite could be true: it is apparent that metal films
cannot be stretched as far as aqueous films, which can be made as thin as 10
nm, whereas metal films usually rupture at 20–80 mm
depending on the type of foam, which is about the diameter of the solid particles.
It can, therefore, be suspected that solid particles destabilize films when
they become too thin rather than stabilize them.
The development of metallic foams looks back on a long history. The first serious attempts to make such foams date back into the 1950s. However, none of the processes available today and in the past have been brought to a level of sophistication comparable with that of polymeric foams. Deficiencies of the various metal-foaming techniques can be found on many levels, namely:
1. J. Banhart,
Prog. Mater. Sci., 47 (1) (2001), in press.
2. P. Åsholt, Metal Foams
and Porous Metal Structures, ed. J. Banhart, M.F. Ashby, and N.A Fleck (Bremen,
Germany: MIT-Verlag,
1999), p. 133.
3. J. Wood, Metal Foams,
ed. J. Banhart and H. Eifert (Bremen, Germany: MIT-Verlag,
1997), p. 31.
4. O. Prakash, H. Sang, and
J.D. Embury, Mater.
Sci. Eng., A199 (1995), p. 195.
5. L.D. Kenny, Mater. Sci.
Forum, 217-222 (1996), p. 1883.
6. A.E. Simone and L.J. Gibson,
Acta
Mater., 46 (1998), p. 3109.
7. J.T. Beals and M.S. Thompson,
J.
Mater. Sci., 32 (1997), p. 3595.
8. T. Miyoshi, in Ref.
2, p. 125.
9. L. Ma, Z. Song, Scripta
Mater., 39 (1998), p. 1523.
10. V. Shapovalov, Porous
and Cellular Materials for Structural Applications, vol. 521, ed. D.S. Schwartz
et al. (Warrendale, PA: MRS,
1998), p. 281.
11. F. Baumgärtner, I. Duarte,
and J. Banhart, Adv. Eng. Mater., 2 (2000), p. 168.
12. I. Duarte and J. Banhart,
Acta
Mater., 48 (2000), p. 2349.
13. J. Banhart and J. Baumeister,
J.
Mater. Sci., 33 (1998), p. 1431.
14. T. Höpler, F. Schörghuber,
and F. Simancík, in Ref. 2, p. 79.
15. J. Baumeister, Sandwich
Construction 5, vol. I, ed. H.R. Meyer-Piening and D. Zenkert (Solihull,
U.K.: EMAS Publishing,
2000), p. 339.
16. J. Banhart et al., Aluminium,
76 (2000), p. 491.
17. W. Seeliger, in Ref.
2, p. 29.
18. J. Banhart et al., German
patent 19,813,176 (1998).
19. V. Gergely and T.W. Clyne,
Adv. Eng. Mater., 2 (2000), p. 175.
20. D. Weaire and S. Hutzler,
The Physics of Foams (Oxford, U.K.: Oxford
University Press, 2000).
21. S.W. Ip, Y. Wang, and J.M.
Toguri, Canadian Metallurgical
Quarterly, 38 (1999), p. 81.
22. E.M. Levin, C.R. Robbins,
and H.F. McMurdie, Phase Diagrams for Ceramists (Columbus, OH: American
Ceramic Society, 1964).
23. T.B. Massalski, Binary
Phase Diagrams (Metals Park, OH: ASM,
1983).
24. Y, Sugimura et al., Acta
Mater., 45 (1997), p. 5245.
25. G. Kaptay, in Ref.
2, p. 141.
26. A. Irretier, P. Weferling,
and J. Banhart, to be published.
27. P. Weigand, Untersuchung
der Einflußfaktoren auf die pulvermetallurgische Herstellung von Aluminiumschäumen
(Bremen, Germany: MIT-Verlag,
1999) [in German].
28. J. Banhart et al., Appl.
Phys. Lett., (submitted October 2000) 29. M.F. Ashby et al., Metal Foams:
a Design Guide (Boston, MA: Butterworth-Heinemann,
2000).
John Banhart is a research scientist at Fraunhofer-Institute for Advanced Materials in Bremen, Germany and a reader in physics at University of Bremen.
For more information, contact John Banhart, Fraunhofer-Institute
for Manufacturing and Advanced Materials, Wiener Str. 12, 28359 Bremen, Germany;
e-mail banhart@telda.net.
Direct questions about this or any other JOM page to jom@tms.org.
| If you would like to comment on the December
2000 issue of JOM,
simply complete the JOM on-line critique form |
|||||
|---|---|---|---|---|---|
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |