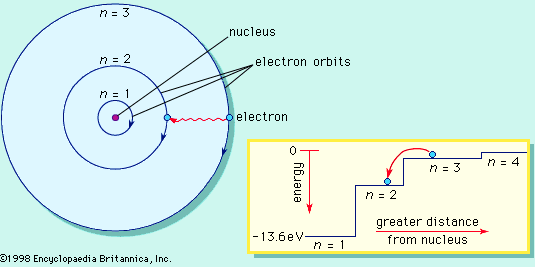
Figure 14. The Bohr atom. The electron travels
in circular orbits that have quantized sizes and energies. Energy is emitted
from the atom when the electron jumps from outer to inner orbit. A transition,
in which an electron jumps from orbit n = 3 to orbit n = 2, produces a photon
of red light with an energy of 1.89 eV and a wavelength of 656 .10-9
m.21
CLOSE
WINDOW