|
METALLURGICAL AND MATERIALS TRANSACTIONS B
|

|
ABSTRACTS
Volume 26B, No. 4, April 1995
This Month Featuring: The 1994 Extraction and Processing Lecture; Hydrometallurgy; Pyrometallurgy; Transport Phenomena; Process Control; Physical Chemistry; Solidification; Solid State Reactions; Welding & Joining. View April 1995 Contents.
|
1994 EXTENSION AND PROCESSING LECTURE
Electricity in the Production of Metals: From Aluminum to Zinc
J.W. EVANS
HYDROMETALLURGY
Kinetics of Pyrite Oxidation in Sodium Carbonate Solutions
V.S.T. CIMINELLI and K. OSSEO-ASARE
The kinetics of pyrite oxidation in sodium carbonate solutions were investigated in a stirred vessel, under temperatures ranging from 50°C to 85°C, oxygen partial pressures from 0 to 1 atm, particle size fractions from -150 + 106 to -38 + 10 µm (-100 + 150 Mesh to -400 Mesh + 10 µm) and pH values of up to 12.5. The rate of the oxidation reaction is described by the following expression:
-dN/dt = SbkpO20.5 [OH-]0.1
where N represents moles of pyrite, S is the surface area of the solid particles, b is a stoichiometric factor, k is an apparent rate constant, pO2 is the oxygen partial pressure, and [OH-] is the hydroxyl ion concentration. The experimental data were fitted by a stochastic model for chemically controlled reactions, represented by the following fractional conversion (X) vs time (t) equation:
(1 - X)-2/3 - 1 = kSTt
The assumption behind this model, i.e., surface heterogeneity leading to preferential dissolution, is supported by the micrographs of reacted pyrite particles, showing pits created by localized dissolution beneath an oxide layer. In addition to the surface texture, the magnitude of the activation energy (60.9 kJ/mol or 14.6 ± 2.7 kcal/mol), the independence of rate on the stirring speed, the inverse relationship between the rate constant and the initial particle diameter, and the fractional reaction orders are also in agreement with a mechanism controlled by chemical reaction.
The Leaching of Galena in Ferric Sulfate Media
J.E. DUTRIZAC and T.T. CHEN
The leaching of galena (PbS) in ferric sulfate media was investigated over the temperature range 55°C to 95°C and for various Fe(SO4)1.5, H2S04 FeSO4 and MgSO4 concentrations. Relatively slow kinetics were consistently observed;in most instances the 1-2/3 - (1-
- (1- )2/3 vs time relationship indicative of a diffusion-controlled reaction was closely obeyed. The diffusioncontrolled kinetics were attributed to the formation of a tenacious layer of PbSO4 and S0 on the surface of the galena. The generation and morphology of the reaction products were systematically determined by scanning electron microscopy and complex growth mechanisms were illustrated. The leaching rate increased rapidly with increasing temperature and the apparent activation energy is 61.2 kJ/mol. The rate increases as the 0.5 power of the ferric ion concentration but is nearly independent of the concentration of the FeSO4 reaction product The rate is insensitive to H2SO4 concentrations <0.1 M but increases at higher acid levels. The presence of neutral sulfates such as MgSO4 decreases the leaching rate to a modest extent.
)2/3 vs time relationship indicative of a diffusion-controlled reaction was closely obeyed. The diffusioncontrolled kinetics were attributed to the formation of a tenacious layer of PbSO4 and S0 on the surface of the galena. The generation and morphology of the reaction products were systematically determined by scanning electron microscopy and complex growth mechanisms were illustrated. The leaching rate increased rapidly with increasing temperature and the apparent activation energy is 61.2 kJ/mol. The rate increases as the 0.5 power of the ferric ion concentration but is nearly independent of the concentration of the FeSO4 reaction product The rate is insensitive to H2SO4 concentrations <0.1 M but increases at higher acid levels. The presence of neutral sulfates such as MgSO4 decreases the leaching rate to a modest extent.
PYROMETALLURGY
The Activity Coefficient of Cobalt Oxide in Silica-Saturated Iron Silicate Slags
ERIC J. GRIMSEY and XULIANG LIU
The solubility of cobalt oxide in silica-saturated iron silicate slags (1.16 to 10.00 wt pct) in equilibrium with cobalt-gold-iron alloys (1.10 to 6.52 wt pct cobalt) and oxygen pressures of 10-9 to 10-10 atm (1 atm = 1.013 x 105 Pa) has been investigated at 1573 K. The activity coefficient of cobalt oxide, 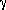 CoO has been calculated relative to pure solid cobalt oxide as standard, namely,
CoO has been calculated relative to pure solid cobalt oxide as standard, namely,
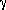 CoO = 0.91 ± 0.09
CoO = 0.91 ± 0.09
and a relationship derived between weight percent cobalt in slag, Co (wt pct), oxygen pressure, pO2, and activity of cobalt relative to liquid cobalt, aCo, namely,
Co(wt pct)= 1.32 x 106pO aCo ± 10pct
aCo ± 10pct
Both errors are calculated at the 95 pct confidence level.
Studies on Kinetics of Low-Temperature Chlorination of ZrO2 by Gaseous Carbon Tetrachloride
P.K. JENA, E.A. BROCCHI, and T.F. VILLELA
Studies on the kinetics of chlorination of ZrO2 powder by carbon tetrachloride vapor in mixture with nitrogen in a low-temperature range of 650 to 825 K at different periods and partial pressures of carbon tetrachloride were carried out. The chlorination results at 650 and 675 K seem to follow a diffusion-controlled reaction model of Jander's type:
[1 - (1 - R)1/3]2 = k1t
where R is the fraction of ZrO2 chlorinated in time t and kl is the rate constant. The approximate activation energy of the process was calculated from k,values at the previously mentioned two temperatures and found to be 278 kJ/mole. For the chlorination in the temperature range of ~ 700 to 750 K, the topochemical reaction model
1 - (1 - R)1/3 = k2t
was followed. The rate constant, k2, was found to be proportional to the partial pressure of carbon tetrachloride. The activation energy of this reaction was calculated to be 154 kJ/mole. In the temperature range of 775 to 825 K, the rate of chlorination was found to be directly proportional to the time of chlorination following Langmuir's Adsorption Isotherm. Because of the very high rate of chlorination and thermodynamic possibility of decomposition of CCI4 above 773 K, the rate-controlling step has been suggested to be the decomposition of the adsorbed complex formed by ZrO2 with carbon and chlorine atoms, obtained from the decomposition of CCI4 vapor. The activation energy of the process was 54 kJ/mole. In view of nearly complete chlorination of ZrO2 by CCI4 in a very short period of about 15 minutes, at a temperature around 800 K and lesser possibility of formation of toxic product gases, the process is recommended for commercial application.
Communication: Phase Relations in the System MgO-SiO2ZrO2 at 1700 K
S.S. PANDIT and K.T. JACOB
TRANSPORT PHENOMENA
Velocity and Turbulence Measurements in a Cylindrical Bath Subject to Centric Bottom Gas Injection
MANABU IGUCHI, TSUNEO KONDOH, ZEN-ICHIRO MORITA, KEIJI NAKAJIMA, KAZUHARU HANAZAKI, TOMOMASA UEMURA, and FUJIO YAMAMOTO
Laser Doppler velocimeter (LDV) measurements were made to clarify the fluid flow behavior in a bath subject to centric bottom gas injection. Correlations of the axial mean velocity and turbulence components in the gas-liquid two-phase flow region, i.e., in the bubbling jet region, were proposed as functions of the inner diameter of nozzle, gas flow rate, and densities of gas and liquid. Measured values of the flow rate, momentum, and kinetic energy of water rising upward were approximated satisfactorily by these empirical correlations. In addition, the Reynolds shear stress was calculated and compared with measured values.
PROCESS CONTROL
Communication: Role of Ore/Carbon Contact and Direct Reduction in the Reduction of Iron Oxide by Carbon
R. HAQUE and H.S. RAY
PHYSICAL CHEMISTRY
Calcium Deoxidation and Nitrogen Distribution in Liquid Nickel Equilibrated with CaO-AI2O3 Slags
SUNG-WOOK CHO and HIDEAKI SUITO
The Wagner s first-order (e ) and second-order (r
) and second-order (r ) interaction parameters between Ca and O in liquid nickel were determined as -1220 and 1.35 x 105, respectively, at 1873 K in the equilibrium experiments between linuid nickel and CaO-AI2O3 slags in an Al2O3 or CaO crucible. The values for e
) interaction parameters between Ca and O in liquid nickel were determined as -1220 and 1.35 x 105, respectively, at 1873 K in the equilibrium experiments between linuid nickel and CaO-AI2O3 slags in an Al2O3 or CaO crucible. The values for e , (- 3060), r
, (- 3060), r (8.47 x 105), r
(8.47 x 105), r (6.76 x 105), and r
(6.76 x 105), and r (6.76 x 105) were also estimated. Nitride capacities, C(N), defined by (mass pct N) · P
(6.76 x 105) were also estimated. Nitride capacities, C(N), defined by (mass pct N) · P /P
/P were obtained by using the present results for nitrogen distribution ratio, LN {=(mass pct N)/[mass pct N]}, and the reported values for the activity of Al2O3.
were obtained by using the present results for nitrogen distribution ratio, LN {=(mass pct N)/[mass pct N]}, and the reported values for the activity of Al2O3.
A Thermodynamic Study of the System CaO + Al2O3 + FexO at 1673 K
V. ESPEJO and M. IWASE
Electrochemical measurements of the solid-oxide galvanic cell:
Mo/Mo+MoO2/ZrO2(MgO)/(CaO+Al2O3
+FexO)+Fe(s)+Ag/Fe
have been conducted at 1673 K in order to obtain the activities of FexO in CaO + Al2O3 + FexO slags. By using the activity data for FexO the isothermal section of the phase diagram for the system CaO + Al2O3 + FexO was derived.
In Situ Combustion Synthesis of Dense Ceramic and Ceramic-Metal Interpenetrating Phase Composites
H.J. FENG and J.J. MOORE
A model exothermic reaction is used to demonstrate the application of simultaneous combustion synthesis, conducted under a consolidating pressure, as a one-step in situ synthesis technique for the production of dense ceramic and ceramic-metal interpenetrating phase composites (IPC). The addition of an excess amount of metal, e.g., Al, and/or a diluent, e.g., Al2O3, lowers the combustion temperature and aids in the refinement of the microstructure, facilitating an increase in compressive strength and elastic modulus. The effects of the important process parameters, e.g., reaction stoichiometry and diluents, green density, pressure, and temperature, on microstructure and properties of these high-performance composites are discussed.
Standard Enthalpies of Formation of Neodymium Alloys, Nd + Me (Me  Ni, Ru, Rh, Pd, Ir, Pt), by High-Temperature Direct Synthesis Calorimetry
Ni, Ru, Rh, Pd, Ir, Pt), by High-Temperature Direct Synthesis Calorimetry
QITI GUO and O.J. KLEPPA
The Standard enthalpies of formation of 14 neodymium alloys have been determined by direct synthesis calorimetry at 1477 ± 2 K. The following values of  H°f (kJ/g atom) are reported: NdNi5, - (26.2 ± 1.1); Nd5Ru2, - (17.2 ± 1.9); NdRu2, - (18.8 ± 1.2); Nd5Rh4, -(59.9 ± 2.5); NdRh, -(64.2 ± 2.0); NdRh2, - (59.9 ± 1.1); NdRh3, - (44.4 ± 1.6); NdPd, -(77.2 ± 2.7); NdPd3, - (73.3 ± 2.3); Nd5Ir3, - (59.7 ± 2.7); Ndlr2, - (67.6 ± 1.5); NdPt, - (104.4 ± 2.6); NdPt2, - (97.9 ± 2.4); and NdPt5, - (55.0 ± 3.1). The results are compared with available literature data for some of the neodymium alloys and with predicted values from the Miedema model.
H°f (kJ/g atom) are reported: NdNi5, - (26.2 ± 1.1); Nd5Ru2, - (17.2 ± 1.9); NdRu2, - (18.8 ± 1.2); Nd5Rh4, -(59.9 ± 2.5); NdRh, -(64.2 ± 2.0); NdRh2, - (59.9 ± 1.1); NdRh3, - (44.4 ± 1.6); NdPd, -(77.2 ± 2.7); NdPd3, - (73.3 ± 2.3); Nd5Ir3, - (59.7 ± 2.7); Ndlr2, - (67.6 ± 1.5); NdPt, - (104.4 ± 2.6); NdPt2, - (97.9 ± 2.4); and NdPt5, - (55.0 ± 3.1). The results are compared with available literature data for some of the neodymium alloys and with predicted values from the Miedema model.
Viscosity of PbO-SiO2 Melts
SURESH K. GUPTA
Viscosities ( ) of PbO-SiO2 melts that contained 25.0 to 48.8 mole pct SiO2 were measured at temperatures 928 to 1273 K by a rotating cylinder method. The data were analyzed in terms of the conventional polymer theory. The results followed Arrhenius behavior in the different temperature ranges despite the general belief of non-Arrhenius behavior of viscosity in slag melts. The calculated activation energies were a function of temperature and composition of the melts. The activation energies for viscous flow, in general, at above approximately 850°C were lower than those below 850°C and varied between 25 and 150 kJ per mole.
) of PbO-SiO2 melts that contained 25.0 to 48.8 mole pct SiO2 were measured at temperatures 928 to 1273 K by a rotating cylinder method. The data were analyzed in terms of the conventional polymer theory. The results followed Arrhenius behavior in the different temperature ranges despite the general belief of non-Arrhenius behavior of viscosity in slag melts. The calculated activation energies were a function of temperature and composition of the melts. The activation energies for viscous flow, in general, at above approximately 850°C were lower than those below 850°C and varied between 25 and 150 kJ per mole.
The Equilibrium Oxygen Pressure over the Cr-Y2O3-YCrO3 Coexistence Measured with the Galvanic Cell Using Stabilized ZrO2 Solid Electrolyte
KEN-ICHI KAWAMURA, TOSHIO MARUYAMA, and KAZUHIRO NAGATA
The equilibrium oxygen pressure over the Cr-Y2O3-YCrO3 coexistence has been measured by the following cells:
Cr, Y2O3, YCrO3|ZrO2|Cr, Cr203[I]
Mn, MnO|ZrO2|Cr, Y2O3,YCrO3[II]
Moreover, the partial electronic conduction parameter, Pe, has been determined simultaneously, as the oxygen partial pressure where the n-type electronic and ionic conductivities are equal in the stabilized ZrO2. The equilibrium oxygen pressure, PO2. over the Cr-Y2O3-YCrO3 coexistence and Pe are expressed as
log (PO2/atm) = 10.6 - 4.39 x 104  ± 0.1 (1385 to 1470 K)
± 0.1 (1385 to 1470 K)
Iog (Pe/atm) = 8.86 - 4.21 x 104  ± 0.3 (1385 to 1470 K)
± 0.3 (1385 to 1470 K)
From the equilibrium oxygen pressure and the standard Gibbs energy of formation of Y2O3, the standard Gibbs energy of formation of YCrO3 is calculated as
 G°f/J mol-1 = -1.58 X 106 + 2.93 X 102 T ± 7 x 103 (1385 to 1470 K)
G°f/J mol-1 = -1.58 X 106 + 2.93 X 102 T ± 7 x 103 (1385 to 1470 K)
Activities of MnO in CaO-SiO2-AI2O3-MnO (<10 Pct)-Fet0(<3 Pct) Slags Saturated with Liquid Iron
HIROKI OHTA and HIDEAKI SIJITO
Activity coefficients of MnO and FetO in CaO-SiO2-AI2O3-MnO(<10 mass pct)-Fe,0(<3 mass pct) slags were determined at 1873 K in an Al2O3 or CaO crucible by using the reported values for the activities of Al2O3 and SiO2 or the analyzed contents of oxygen. The activity coefficients of MnO and FetO were found to be constant in the studied concentration range of MnO and FetO. The former increased with an increase in the CaO content, while the latter increased with an increase in the SiO2 content.
Extensions of a Structural Model for Binary Silicate Systems
ANTONIO ROMERO-SERRANO and ARTHUR D. PELTON
Our earlier structural model for binary silicate melts and glasses is extended. More general expressions for the enthalpy and nonconfigurational entropy are given. Expressions for partial thermodynamic properties are derived. A least-squares optimization program permits all available thermodynamic and phase diagram data to be optimized simultaneously within experimental error limits. The model is extended from MO-SiO2 to M2O-SiO2 solutions. Data in acidic melts is well represented for the Na2O-SiO2 system. Examples of optimizations for the MnO-SiO2, CaO-SiO2, and Na2O-SiO2 systems are presented. The model is extended to include sulfide ion. Good predictions of sulfide capacities are obtained
Kinetics of the Reaction of CH4 Gas with Liquid Iron
K. SEKINO, T. NAGASAKA, and R.J. FRUEHAN
In iron bath smelting and other processes that use coal, the effective use of volatile matter can improve the energy efficiency of the process. The reaction of simulated volatile (CH4) with iron was studied. The rate of carburization of liquid iron by CH4 gas was measured between 1400°C and 1700°C under conditions for which the effect of mass transfer can be corrected with reasonable accuracy. The rate was measured for partial pressures of CH4 in Ar in the range of 0.02 to 0.06 atm and sulfur contents in the metal from 0.0006 to 0.5 mass pct. The results indicate that the rate of carburization may be controlled by the dissociation of CH4 on the surface. Sulfur was found to decrease the rate, and the residual rate phenomenon was observed for high sulfur contents. The rate constant may be represented by the following equation:

where k°, kr, KS and aS are the bare surface rate constant, residual rate constant, adsorption coefficient for sulfur, and activity of sulfur in the metal, respectively. The second term in the rate equation represents the rate of dissociation on the adsorbed sulfur. The rate constants and adsorption coefficient were determined as functions of temperature to be
log k° =  + 2.95 (mole/cm2 s atm)
+ 2.95 (mole/cm2 s atm)
log kr =  + 3.45 (mole/cm2 s atm)
+ 3.45 (mole/cm2 s atm)
log Ks =  + 1.04
+ 1.04
Sulfide Capacities of Fayalite-Base Slags
S.R. SIMEONOV, R. SRIDHAR, and I.M. TOGURI
The sulfide capacities of fayalite-base slags were measured by a gas-slag equilibration technique under controlled oxygen and sulfur potentials similar to those encountered in the pyro metallurgical processing of nonferrous metals. The oxygen pressure range was from 10-9.5 to 10-11 MPa and the sulfur pressure range from 10-3 to 10-4.5 MPa, over a temperature range of 1473 to 1623 K. The slags studied were FeO-SiO2 at silica saturation and those with addition of CaO, MgO, and Al2O3 to determine their effect on sulfide capacities. For these slags, the sulfide capacities were found to vary from 10-3.3 to 10-5. The sulfide capacities increased with increasing temperature from 1473 to 1623 K. A comparison of the reported plant data on sulfur content of industrial slags shows good agreement with the present experimental results. The present data will be useful in estimating metal losses in slag due to metal sulfide entrainment in nonferrous smelters.
Kinetics of Oxidation of Carbon in Liquid Iron-Carbon-Silicon-Manganese-Sulfur Alloys by Carbon Dioxide in Nitrogen
HAIPING SUN and ROBERT D. PEHEKE
The oxidation of carbon with the simultaneous oxidation of silicon, manganese, and iron of liquid alloys by carbon dioxide in nitrogen and the absorption of oxygen by the alloys from the gas were studied using 1-g liquid iron droplets levitated in a stream of the gas at 1575°C to 1715°C. Oxidation of carbon was favored over oxidation of silicon and manganese when cast iron (3.35 pct C, 2.0 pct Si, 0.36 pct Mn, and 0.05 pct S) reacted with CO2/N2 gas at 1635°C. An increase in the flow rate of CO2/N2 gas increased the decarburization rate of cast iron. The rate of carbon oxidation by this gas mixture was found to be independent of temperature and alloying element concentrations (in the range of silicon = 0 to 2.0 pct manganese = 0 to 0.36 pct and sulfur = 0 to 0.5 pct) within the temperature range of the present study. Based on the results of a kinetic analysis, diffusion of CO2 in the boundary layer of the gas phase was found to be the rate-limiting step for the reactions during the earlier period of the reaction whenthe contents of carbon, silicon, and manganese are higher. However, the limiting step changed to diffusion of the elements in the metal phase during the middle period of the reaction and then to the diffusion of CO in the gas phase during the later period of the reaction when the content of the elements in the metal were relatively low. For the simultaneous oxidation reactions of several elements in the metal, however, the diffusion of CO2 in the gas phase is the primary limiting step of the reaction rate for the oxidation of carbon during the later period of reaction.
Effect of Factors on the Extraction of Boron from Slags
PEIXIN ZHANG and ZHITONG SUI
The effects of slag composition, additive agent, and heat treatment on the crystal morphologies, the crystallization behavior, and the efficiency of extraction of boron (EEB) from slags were investigated by chemical analysis, polarization microscope, and X-ray diffraction (XRD) as well as differential thermal analysis (DTA). The EEB varied with the slag composition. The farther the slag composition deviated from the line between 2MgO · B2O3 and 2MgO · SiO2 in the MgO-B2O3-SiO2 system, the lower the EEB. The EEB was directly related to the precipitating characteristics of the boron component in the slags. The EEB was high if the boron component existed in the form of a crystalline phase, otherwise the EEB was low when boron was in the form of an amorphous phase. The EEB from MgO-AI203-CaO-B2O3-SiO2 slag varied with the temperature of heat treatment; the highest EEB appeared at 1100°C. The EEB and the crystallinities were increased by addition of TiO2 and MOX (M = Mg, Ca, Fe, Si). The effect of MOX was more notable than that of TiO2.
SOLIDIFICATION
Study of Meniscus Behavior and Surface Properties during Casting in a High-Frequency Magnetic Field
TINGJU LI, SHINJI NAGAYA, KENSUKE SASSA, and SHIGEO ASAI
Surface quality of continuously cast metals can be improved by imposing a continuous high-frequency magnetic field from the outside of a mold. Newly proposed concepts of "soft contacting solidification" and "slow cooling solidification," which is tightly related to the mechanism of improving surface quality, were confirmed in model experiments by using molten gallium and tin. The meniscus motion of the molten gallium accompanied by a mold oscillation and magnetic pressure was measured by a laser level sensor. The shape variation of a meniscus and the process of ripple formation in an oscillation cycle were directly visualized by an optical fiberscope camera. Moreover, molten tin was continuously cast and the relationship between the surface quality and the meniscus motion was studied. A mechanical model for predicting the space between the oscillation marks is proposed. The casting process using intermittent high frequency magnetic feld was developed. New functions of this field were investigated regarding the control of initial solidification. It was found that the surface quality of the continuously cast metal can be improved by the intermittent high-frequency magnetic field as well as the continuous high-frequency magnetic field.
Heat Transfer and Microstructure during the Early Stages of Metal Solidification
C.A. MUOJEKWU, I.V. SAMARASEKERA, and J.K. BRIMACOMBE
Transient heat transfer in the early stages of solid)fication of an alloy on a water-cooled chill and the consequent evolution of microstructure, quantified in terms of secondary dendrite arm spacing (SDAS), have been studied. Based on dip tests of the chill, instrumented with thermocouples, into Al-Si alloys, the influence of process variables such as mold surface roughness, mold material, metal superheat, alloy composition, and lubricant on heat transfer and cast structure has been determined. The heat flux between the solidifying metal and substrate, computed from measurements of transient temperature in the chill by the inverse heat-transfer technique, ranged from low values of 0.3 to 0.4 MW/m2 to peak values of 0.95 to 2.0 MW/m2. A one dimensional, implicit, finite-difference model was applied to compute heat-transfer coefficients, which ranged from 0.45 to 4.0 kW/m2 °C, and local cooling rates of 10°C/s to 100°C/s near the chill surface, as well as growth of the solidifying shell. Near the chill surface, the SDAS varied from 12 to 22 µm while at 20 mm from the chill, values of up to 80 µm were measured. Although the SDAS depended on the cooling rate and local solidification time, it was also found to be a direct function of the heat-transfer coefficient at distances very near to the casting/chill interface. A three-stage empirical heat-flux model based on the thermophysical properties of the mold and casting has been proposed for the simulation of the mold/casting boundary condition during solidification. The applicability of the various models proposed in the literature relating the SDAS to heat-transfer parameters has been evaluated and the extension of these models to continuous casting processes pursued.
SOLID STATE REACTIONS
Communication: Reduction Behavior of Ilmenite with Carbon at 1240°C
SURESH K. GUPTA and P. GRIEVISON
WELDING and JOINING
Heat and Metal Transfer in Gas Metal Arc Welding Using Argon and Helium
P.G. JÖNSSON, T.W. EAGAR, and J. SZEKELY
This article describes a theoretical investigation on the arc parameters and metal transfer in gas metal arc welding (GMAW) of mild steel using argon and helium shielding gases. Major differences in the predicted arc parameters were determined to be due to large differences in thermophysical properties Various findings from the study include that an arc cannot be struck in a pure helium atmosphere without the assistance of metal vapor, that a strong electromagnetic cathode force affects the fluid flow and heat transfer in the helium arc, providing a possible explanation for the experimentally observed globular transfer mode and that the tapering of the electrode in an argon arc is caused by electron condensation on the side of the electrode.
Direct questions about this or any other Metallurgical and Materials Transactions page to mettrans@andrew.cmu.edu.

 - (1-
- (1-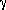 CoO has been calculated relative to pure solid cobalt oxide as standard, namely,
CoO has been calculated relative to pure solid cobalt oxide as standard, namely,
 aCo ± 10pct
aCo ± 10pct ) and second-order (r
) and second-order (r , (- 3060), r
, (- 3060), r (6.76 x 105), and r
(6.76 x 105), and r (6.76 x 105) were also estimated. Nitride capacities, C(N), defined by (mass pct N) · P
(6.76 x 105) were also estimated. Nitride capacities, C(N), defined by (mass pct N) · P /P
/P were obtained by using the present results for nitrogen distribution ratio, LN {=(mass pct N)/[mass pct N]}, and the reported values for the activity of Al2O3.
were obtained by using the present results for nitrogen distribution ratio, LN {=(mass pct N)/[mass pct N]}, and the reported values for the activity of Al2O3.
 Ni, Ru, Rh, Pd, Ir, Pt), by High-Temperature Direct Synthesis Calorimetry
Ni, Ru, Rh, Pd, Ir, Pt), by High-Temperature Direct Synthesis Calorimetry H°f (kJ/g atom) are reported: NdNi5, - (26.2 ± 1.1); Nd5Ru2, - (17.2 ± 1.9); NdRu2, - (18.8 ± 1.2); Nd5Rh4, -(59.9 ± 2.5); NdRh, -(64.2 ± 2.0); NdRh2, - (59.9 ± 1.1); NdRh3, - (44.4 ± 1.6); NdPd, -(77.2 ± 2.7); NdPd3, - (73.3 ± 2.3); Nd5Ir3, - (59.7 ± 2.7); Ndlr2, - (67.6 ± 1.5); NdPt, - (104.4 ± 2.6); NdPt2, - (97.9 ± 2.4); and NdPt5, - (55.0 ± 3.1). The results are compared with available literature data for some of the neodymium alloys and with predicted values from the Miedema model.
H°f (kJ/g atom) are reported: NdNi5, - (26.2 ± 1.1); Nd5Ru2, - (17.2 ± 1.9); NdRu2, - (18.8 ± 1.2); Nd5Rh4, -(59.9 ± 2.5); NdRh, -(64.2 ± 2.0); NdRh2, - (59.9 ± 1.1); NdRh3, - (44.4 ± 1.6); NdPd, -(77.2 ± 2.7); NdPd3, - (73.3 ± 2.3); Nd5Ir3, - (59.7 ± 2.7); Ndlr2, - (67.6 ± 1.5); NdPt, - (104.4 ± 2.6); NdPt2, - (97.9 ± 2.4); and NdPt5, - (55.0 ± 3.1). The results are compared with available literature data for some of the neodymium alloys and with predicted values from the Miedema model.
 ) of PbO-SiO2 melts that contained 25.0 to 48.8 mole pct SiO2 were measured at temperatures 928 to 1273 K by a rotating cylinder method. The data were analyzed in terms of the conventional polymer theory. The results followed Arrhenius behavior in the different temperature ranges despite the general belief of non-Arrhenius behavior of viscosity in slag melts. The calculated activation energies were a function of temperature and composition of the melts. The activation energies for viscous flow, in general, at above approximately 850°C were lower than those below 850°C and varied between 25 and 150 kJ per mole.
) of PbO-SiO2 melts that contained 25.0 to 48.8 mole pct SiO2 were measured at temperatures 928 to 1273 K by a rotating cylinder method. The data were analyzed in terms of the conventional polymer theory. The results followed Arrhenius behavior in the different temperature ranges despite the general belief of non-Arrhenius behavior of viscosity in slag melts. The calculated activation energies were a function of temperature and composition of the melts. The activation energies for viscous flow, in general, at above approximately 850°C were lower than those below 850°C and varied between 25 and 150 kJ per mole.
 ± 0.1 (1385 to 1470 K)
± 0.1 (1385 to 1470 K)
 + 2.95 (mole/cm2 s atm)
+ 2.95 (mole/cm2 s atm)
 + 3.45 (mole/cm2 s atm)
+ 3.45 (mole/cm2 s atm)
 + 1.04
+ 1.04