PYROMETALLURGY
Extraction of Tantalum and Niobium from Tin Slags by Chlorination and Carbochlorination
I. GABALLAH, E. ALLAIN, and M. DJONA
Chlorination and carbochlorination of tantalum and niobium low-grade concentrate (LGC) and high-grade concentrate (HGC), obtained by leaching of tin slag, were studied using Cl2 + N2 and Cl2 + CO + N2 gas mixtures. Thermogravimetric analysis and conventional boat experiments were performed between 200°C and 1000°C. Chemical analysis, X-ray diffraction (XRD), and scanning electron microscopy (SEM) were used to characterize the samples and reaction products. Chlorination of LGC led to the recovery of about 95 pct of tantalum and niobium compounds at 1000°C. However, the tantalum and niobium chlorinated compounds were contaminated by chlorides of Fe, Mn, etc. For HGC, chlorination at 1000°C allowed the extraction of about 84 and 65 pct of the niobium and tantalum compounds, respectively. The recovered condensates were composed of pure tantalum and niobium chlorinated compounds. The apparent activation energies Ea for the chlorination of LGC and HGC, between 850°C and 1000°C, were 166 and 293 kJ/mole, respectively. At temperatures lower than 650°C, the apparent activation energies for the LGC and HGC carbochlorination were 116 and 103 kJ/mole, respectively. Total extraction of the tantalum and niobium compounds was achieved by the carbochlorination of the LGC at 1000°C. The generated tantalum and niobium chlorinated compounds were contaminated by the chlorides of Fe, Mn, Al, and Ca. The carbochlorination of the HGC at 500°C allowed complete extraction and recovery of pure tantalum and niobium compounds. These results confirm the importance of obtaining an HGC from tin slag before its subsequent chlorination. The carbochlorination of such a concentrate could be an efficient process for the recovery of relatively pure tantalum and niobium chlorinated compounds at low temperatures.
Kinetics of Chloridization of Nickel Oxide with Gaseous Hydrogen Chloride
S.B. KANUNGO and S.K. MISHRA
The kinetics of chloridization of finely divided as well as granulated nickel oxide by gaseous HCl were studied in the temperature range 150°C to 400°C. The rate of chloridization depended upon temperature, partial pressure of HCl, and granule size. The conversion of NiO to NiCl2 follows the logarithmic rate law or a pore-blocking model, which is attributed to the large increase in molar volume of the product phase. The blocked pores decreased the diffusion of gaseous reactant through the product layer. This observation has been supported by the low value of activation energy, the high order of reaction with respect to the partial pressure of HCl (g), and a slope value of -2 for the log-log plot of rate constant vs grain size. The morphological characteristics of both unreacted and chloridized nickel oxide have been studied with the help of a scanning electron microscope.
Kinetics of Chloridization of Nickel-Bearing Lateritic Iron Ore by Hydrogen Chloride Gas
S.B. KANUNGO and S.K. MISHRA
The selective chloridization of nickel in a lateritic iron ore by gaseous HCl is based on the principle of relative thermal stability of iron and nickel chlorides. This aspect has been discussed with differential thermal analysis (DTA) and thermogravimetric (TG) data of the hydrated chlorides of iron and nickel. The kinetics of chloridization of nickel in a lateritic nickel ore from Orissa, India, have been studied by using both pure HCl (g) and the HCl (g) + N2 mixture. The sharp decrease in the rate of chloridization of nickel at temperatures above 250°C is attributed to the rapid decomposition of molten ferric chloride hydrate (FeCl3 · 3H2O), which blocks the pores of the reactant solid. Therefore, kinetics of chloridization follow both the pore-blocking model (logarithmic rate law) and diffusion-controlled mechanisms. Very low values of apparent activation energy and effective diffusivity derived from the rate constants of the diffusion-controlled process suggest that diffusion of HCl (g) takes place either in a dissolved state in the molten ferric chloride (at 100°C to 150°C) or through cracks and fissures formed on the surface due to rapid decomposition of ferric chloride at 200°C to 250°C. Because of the complexity of the reaction system, the rate of chloridization of nickel is almost independent of grain size.
Interfacial Phenomena in the Liquid Copper-Calcium Ferrite Slag System
T. SAKAI, S.W. IP, and J.M. TOGURI
The surface and interfacial tensions of the copper-calcium ferrite slag system were determined at 1573 K as a function of oxygen and sulfur pressures and CaO content in the slag. The effect of a 2.9 mass pct SiO2 addition to the calcium ferrite slag was also investigated. The measurements were carried out using the sessile drop technique with a high-temperature X-ray setup. The surface tension of copper was found to be a strong function of oxygen and sulfur partial pressures. The surface tension of the calcium ferrite slag decreased slightly with CaO addition. On the other hand, CaO had a negligible effect on the interfacial tension of the system. Surface tension of the slag and the interfacial tension were influenced by the oxygen partial pressure in the system. Sulfur partial pressure also altered the interfacial tension between the copper and the ferrite slag. Based on the experimental data, filming and flotation coefficients were calculated to predict the entrainment behavior of copper, by bubble attachment in the ferrite slag.
Communication: Reduction of Ilmenite in a Nonequilibrium Hydrogen Plasma
D.E. BULLARD and D.C. LYNCH
TRANSPORT PHENOMENA
Development of a Multineedle Electroresistivity Probe for Measuring Bubble Characteristics in Molten Metal Baths
MANABU IGUCHI, TADATOSHI NAKATANI, and HIROTOSHI KAWABATA
Precise information on the behavior of rising bubbles in molten metals is of essential importance for the enhancement of current metal-refining processes as well as the development of new refining processes agitated by gas injection. In particular, the total interfacial area between bubbles and molten metal is a key parameter, but it cannot be evaluated unless the shape of bubbles is given. Getting information on the shape of rising bubbles has been very difficult for nontransparent liquids. In this study, we developed a multineedle electroresistivity probe to detect the shape of bubbles rising in molten metal baths. The accuracy of the presently developed probe was examined for water-air and Wood's metal-He systems using a conventional two-needle electroresistivity probe and a high-speed video camera.
The Shape of Bubbles Rising near the Nozzle Exit in Molten Metal Baths
MANABU IGUCHI, TADATOSHI NAKATANI, and HIROHIKO TOKUNAGA
A previously developed multineedle electroresistivity probe was used to investigate the shape of bubbles generated at the exit of a central single-hole bottom nozzle in molten Wood's metal and mercury baths. This probe is capable of detecting the vertical cross section of rising bubbles. The shape of bubbles just after the detachment from the nozzle exit was correlated as a function of a modified Reynolds number and a modified Weber number. Furthermore, the relations between the shape of bubbles and the radial distributions of bubble characteristics specified by gas holdup, bubble frequency, etc. were derived. As a result, it is possible to predict the shape of the bubbles by measuring the bubble characteristics with a conventional two-needle electroresistivity probe.
PROCESS CONTROL
Improvement of Properties of BSCCO Superconductor Tapes with Thermal Processing
H.-J. LIM and J.G. BYRNE
High-Tc BSCCO superconductor tapes were prepared by the powder-in-tube (PIT) method. Some tapes involved partial melting followed by slow cooling, and some involved partial melting followed by rapid quenching. X-ray diffraction showed the presence of BSCCO phases and secondary phases in all the materials. Careful control of the cooling rate (0.1°C/min) after the partial melting at 850°C for 0.5 hours improved the critical current densities and basal texture alignment compared to the rapidly quenched tapes. Direct current (DC) magnetic susceptibility measurements were used to follow the transition at Tc. Critical current density, Jc, values were estimated from DC magnetic hysteresis loops.
PHYSICAL CHEMISTRY
Distribution Behavior of Cobalt, Selenium, and Tellurium between Nickel-Copper-Iron Matte and Silica-Saturated Iron Silicate Slag
N. CHOI and W.D. CHO
The distribution coefficients (Dx) of cobalt, selenium, and tellurium between nickel-copper-iron matte and silica-saturated iron silicate slag were determined as a function of matte and slag compositions, temperature, and the partial pressure of oxygen. The effect of slag additives, such as CaO, MgO, and Al2O3, on the distribution behavior of the minor elements was also investigated. Analysis of the data indicated that DCo, DSe, and DTe were strongly dependent on the matte grade and slag additives. The effect of slag additives on the solubility of Co, Se, and Te in slag was discussed in terms of various experimental conditions. Cobalt distribution coefficients were found to decrease with increasing oxygen partial pressure, indicating the oxidic dissolution of cobalt in the slag. Based on the experimental results and available thermodynamic data, the activity coefficients of CoS in the nickel-copper-iron matte were estimated as a function of mole fraction of FeS in the matte at 1250°C. Meanwhile, the distribution coefficients of both selenium and tellurium increased when raising the partial pressure of oxygen, implying that there was molecular dissolution of selenium and tellurium in the slag within the oxygen partial-pressure range investigated in this study.
A New Generation Solution Model for Predicting Thermodynamic Properties of a Multicomponent System from Binaries
KUO-CHIH CHOU and SHOU-KUN WEI
During the past 3 decades, all solution models that were used to predict thermodynamic properties of a multicomponent system from binaries improperly assumed that the selected binary compositions in a model are independent of the practical system to be treated. This assumption causes problems both in symmetrical and asymmetrical models. In this article, a new solution model has been suggested, which gets rid of this traditional way and assumes that the selected binary compositions should be closely related to the system considered. After introducing a new concept, the "similarity coefficient," the relation between the selected binary compositions and the composition of the multicomponent system is established and a new model is generated. This new generation model is more reasonable in theoretical considerations, more reliable in practical use, and more realistic in computerization for estimating thermodynamic properties and calculating phase diagrams in a multicomponent system.
Phase Equilibria in the Titanium-Oxygen System
D.C. LYNCH and D.E. BULLARD
Data in the literature on the Magneli oxides of titanium have been critically evaluated and equations have been developed from these data for the standard-state Gibbs energy of formation of the following oxides: Ti4O7, Ti5O9, Ti6O11, Ti8O15, and Ti9O17. Examination of those data yielded the following:

 (TiO7/4) = -845.26 + 0.1490
(TiO7/4) = -845.26 + 0.1490  T kJ/mol, 1150 K < T < 1304 K
T kJ/mol, 1150 K < T < 1304 K

 (TiO9/5) = -867.04 + 0.1564
(TiO9/5) = -867.04 + 0.1564  T kJ/mol, 1150 K < T < 1373 K
T kJ/mol, 1150 K < T < 1373 K

 (TiO11/6) = -879.58 + 0.1600
(TiO11/6) = -879.58 + 0.1600  T kJ/mol, 1150 K < T < 1304 K
T kJ/mol, 1150 K < T < 1304 K

 (TiO15/8) = -894.79 + 0.1642
(TiO15/8) = -894.79 + 0.1642  T kJ/mol, 1150 K < T < 1304 K
T kJ/mol, 1150 K < T < 1304 K

 (TiO17/9) = -900.36 + 0.1600
(TiO17/9) = -900.36 + 0.1600  T kJ/mol, 1150 K < T < 1304 K
T kJ/mol, 1150 K < T < 1304 K
Similar equations for Ti16O31 and Ti50O99 have been estimated from their respective data at 1304 K. The result of that analysis has lead to the following equations:

 (TiO31/16) = -916.93 + 0.1703
(TiO31/16) = -916.93 + 0.1703  T kJ/mol, T
T kJ/mol, T  1304 K
1304 K

 (TiO99/50) = -935.63 + 0.1776
(TiO99/50) = -935.63 + 0.1776  T kJ/mol, T
T kJ/mol, T  1304 K
1304 K
These equations, along with data in the literature, have been used to construct the Ti-O stability diagram.
Absorption Kinetics and Mechanisms of Carbon Monoxide in Liquid Niobium
H.G. PARK and REZA ABBASCHIAN
Absorption kinetics and mechanisms of carbon monoxide in liquid niobium were investigated in the temperature range of 2700 to 3000 K in samples levitated in a CO/Ar stream. The carbon and oxygen dissolution in liquid niobium from CO gas is an exothermic process, and the solubilities of carbon and oxygen (Ce, Oe in at. pct) are related to temperature (T in kelvin) and partial pressure of CO (P in atm) as follows:
in atm) as follows:
ln Ce + ln Oe - P = 2.32
= 2.32  104/T - 2.35
104/T - 2.35
The reaction CO 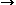 [C] + [O], which occurs along with the evaporation of niobium oxide during the absorption of carbon and oxygen, is a second-order process with a first-order dependence on C and O concentrations, respectively. The absorption mechanism implies that the overall reaction rate is controlled by the substep of dissociation of surface-adsorbed CO molecules. The rate equations for C and O absorption are given as follows:
[C] + [O], which occurs along with the evaporation of niobium oxide during the absorption of carbon and oxygen, is a second-order process with a first-order dependence on C and O concentrations, respectively. The absorption mechanism implies that the overall reaction rate is controlled by the substep of dissociation of surface-adsorbed CO molecules. The rate equations for C and O absorption are given as follows:
(dC/dt) = (l/0.76)(dO/dt) = 12.1(A/V esp (-26,700/T)(Ce Oe - CO)
where C and O are carbon and oxygen concentrations in atomic percent, respectively, A is the surface area of the sample in cm2, and V is the volume in cm3.
Surface Tension and Wettability Studies of Liquid Fe-Ni-O Alloys
A. SHARAN and A.W. CRAMB
Surface tensions of iron-nickel alloys were measured as a function of oxygen potential at 1550°C using the sessile drop technique. The surface tension of pure liquid nickel and iron-nickel alloys was measured at a total pressure of 1 atmosphere under varying CO2/CO ratios. An increase in the oxygen potential in the gas phase was found to correspond to a decrease in surface tension of pure nickel and iron-nickel alloys, indicating that oxygen is surface active in both liquid nickel and iron-nickel alloys. At low oxygen potentials, nickel additions to liquid iron were found to cause small decreases in alloy surface tensions; however, at higher oxygen potentials, the surface tension of the alloy exhibited a minimum value as nickel was added to iron. The adsorption coefficients of oxygen in liquid iron-nickel alloys and pure liquid nickel were determined from the surface-tension data using Belton's analysis, and were found to be similar to those calculated from kinetic studies. Wettability of iron-nickel alloys on an alumina substrate was studied through contact-angle measurements. At a constant alloy nickel content, the contact angle between the alloy and alumina decreased with increased oxygen potential in the gas phase.
Enthalpies of Formation of Liquid (Copper + Manganese) Alloys
M.A. TURCHANIN and I.V. NIKOLAENKO
The enthalpies of formation of liquid (Cu + Mn) alloys were measured in the isoperibolic heat-flux calorimeter at 1573 K in the entire range of compositions. The integral molar enthalpy of mixing was found to be negative in the range of molar fractions 0 < xMn < 0.31, with  H(min) = -0.69 ± 0.27 kJ mol-1 at xMn = 0.12, and positive in the range 0.31 < xMn < 1, with
H(min) = -0.69 ± 0.27 kJ mol-1 at xMn = 0.12, and positive in the range 0.31 < xMn < 1, with  H(max) = 3.67 ± 0.36 kJ mol-1 at xMn = 0.75. Limiting partial molar enthalpies of manganese and copper were calculated as
H(max) = 3.67 ± 0.36 kJ mol-1 at xMn = 0.75. Limiting partial molar enthalpies of manganese and copper were calculated as 
 = -18.0 ± 6.6 kJ mol-1 and
= -18.0 ± 6.6 kJ mol-1 and 
 = 29.1 ± 4.9 kJ mol-1, respectively. The results are discussed in comparison with the thermodynamic data available in the literature and the equilibrium phase diagram.
= 29.1 ± 4.9 kJ mol-1, respectively. The results are discussed in comparison with the thermodynamic data available in the literature and the equilibrium phase diagram.
Communication: Studies on the Thermodynamic Stability of Silver Selenide
A. NASAR and M. SHAMSUDDIN
SOLIDIFICATION
Modeling of Macrosegregation Due to Thermosolutal Convection and Contraction-Driven Flow in Direct Chill Continuous Casting of an Al-Cu Round Ingot
A.V. REDDY and C. BECKERMANN
Macrosegregation in direct chill (DC) continuous casting of an Al-4.5 wt pct Cu round ingot is numerically simulated. The model incorporates descriptions of heat transfer, solute redistribution, and melt convection on the system scale with microscopic relations for grain growth, solutal undercooling, and microsegregation. Simulations are conducted to study the effects of mushy zone permeability, thermosolutal convection, and solidification contraction on the macrosegregation pattern in a DC casting. The results indicate that centerline segregation can be either positive or negative, depending upon the grain density in and permeability of the mush. In addition, it is shown that the flow induced by solidification contraction not only causes inverse segregation at the ingot surface, but also has a significant influence on the macrosegregation across the central portion of the ingot. A comparison with temperature and macrosegregation patterns measured in a previous experiment shows reasonable agreement. Several areas for future model improvements are identified.
MATERIALS PROCESSING
Characterization of the Flow in the Molten Metal Sump during Direct Chill Aluminum Casting
JASON M. REESE
A recent analytical model for the liquid aluminum flow in a direct chill (DC) casting sump has been investigated and the scaling coefficients evaluated. The magnitudes of flow-field features, such as the depth of the temperature stratification in the sump and the velocity of the metal in the thermal boundary layer close to the solidification front, have been calculated. The results broadly agree with recent full numerical calculations of the flow in the sump. The variation of these essential flow features has been investigated across a range of typical ingot sizes, casting speeds, and superheats, and critical macro-casting-parameter combinations have been identified. The limitations of the model are discussed and the possible effects the identified structure has on macrosegregation are briefly explored. Finally, the influence on the flow field of the method of feeding the ingot is investigated, and it is concluded that the model and these results are not invalidated if the feeding is nonuniform over the top surface of the sump.
SURFACE TREATMENT
Analysis of the Laser-Cladding Process for Stellite on Steel
A. FRENK, M. VANDYOUSSEFI, J.-D. WAGNIÈERE, A. ZRYD, and W. KURZ
Laser-cladding experiments have been performed with STELLITE 6 powder on mild steel substrates, using a 1.5 kW linearly polarized continuous wave CO2 laser as a heat source. The clad height, the mass efficiency, the dimensions of the melt pool, as well as the global absorptivity, were measured as functions of the powder feed rate and the scanning speed. A quantitative analytical model of the process is proposed, based on the overall mass and energy balance. It allows the calculation of the mass efficiency and of the global absorptivity, taking into account the incorporation of the powder into the melt pool as well as the energy absorbed by the powder jet and the substrate. It successfully explains the experimental results and demonstrates the role played by the melt pool inclination with respect to the substrate. A processing diagram is given to find rapidly the optimal laser treatment conditions and the desired clad height. It is discussed with respect to the other limiting conditions of the process, the geometrical maximum powder efficiency, the porosity, the dilution, and the maximum power of the laser installation.
MATHEMATICAL MODELING
Mathematical Modeling of Three-Dimensional Heat and Fluid Flow in a Moving Gas Metal Arc Weld Pool
M. USHIO and C.S. WU
Mathematical models capable of accurate prediction of the weld bead and weld pool geometry in gas metal arc (GMA) welding processes would be valuable for rapid development of welding procedures and empirical equations for control algorithms in automated welding applications. This article introduces a three-dimensional (3-D) model for heat and fluid flow in a moving GMA weld pool. The model takes the mass, momentum, and heat transfer of filler metal droplets into consideration and quantitatively analyzes their effects on the weld bead shape and weld pool geometry. The algorithm for calculating the weld reinforcement and weld pool surface deformation has been proved to be effective. Difficulties associated with the irregular shape of the weld bead and weld pool surface have been successfully overcome by adopting a boundary-fitted nonorthogonal coordinate system. It is found that the size and profile of the weld pool are strongly influenced by the volume of molten wire, impact of droplets, and heat content of droplets. Good agreement is demonstrated between predicted weld dimensions and experimently measured ones for bead-on-plate GMA welds on mild steel plate.
Direct questions about this or any other Metallurgical and Materials Transactions page to mettrans@andrew.cmu.edu.


 (TiO7/4) = -845.26 + 0.1490
(TiO7/4) = -845.26 + 0.1490  T kJ/mol, 1150 K < T < 1304 K
T kJ/mol, 1150 K < T < 1304 K 1304 K
1304 K in atm) as follows:
in atm) as follows:
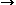 [C] + [O], which occurs along with the evaporation of niobium oxide during the absorption of carbon and oxygen, is a second-order process with a first-order dependence on C and O concentrations, respectively. The absorption mechanism implies that the overall reaction rate is controlled by the substep of dissociation of surface-adsorbed CO molecules. The rate equations for C and O absorption are given as follows:
[C] + [O], which occurs along with the evaporation of niobium oxide during the absorption of carbon and oxygen, is a second-order process with a first-order dependence on C and O concentrations, respectively. The absorption mechanism implies that the overall reaction rate is controlled by the substep of dissociation of surface-adsorbed CO molecules. The rate equations for C and O absorption are given as follows:
 = -18.0 ± 6.6 kJ mol-1 and
= -18.0 ± 6.6 kJ mol-1 and  = 29.1 ± 4.9 kJ mol-1, respectively. The results are discussed in comparison with the thermodynamic data available in the literature and the equilibrium phase diagram.
= 29.1 ± 4.9 kJ mol-1, respectively. The results are discussed in comparison with the thermodynamic data available in the literature and the equilibrium phase diagram.