|
METALLURGICAL AND MATERIALS TRANSACTIONS B
|

|
ABSTRACTS
Volume 29B, No. 4, August 1998
This Month Featuring: Pyrometallurgy; Electrometallurgy; Transport Phenomena; Process Control; Physical Chemistry; Transport Phenomena; Process Control; Physical Chemistry; Solidification; Materials Processing; Welding & Joining; Mathematical Modeling. View August 1998 Contents.
|
PYROMETALLURGY
Kinetics of Carbochlorination of Chromium (III) Oxide
N. KANARI, B.R. REDDY, and I. GABALLAH
Kinetics of the carbochlorination of Cr2O3 has been studied with Cl2 + CO gas mixtures between 500°C to 900°C using thermogravimetric analysis. The apparent activation energy is about 100 kJ/mol. Mathematical fitting of the experimental data suggests that the shrinking sphere model is the most adequate to describe the carbochlorination mechanism of chromium oxide and that is controlled by the chemical reaction. In the temperature range of 550°C to 800°C, the reaction order is about 1.34 and is independent of temperature. Changing the Cl2 + CO content from 15 to 100 pct increases the reaction rate and does not affect the reaction mechanism. Similarly, changing the ratio of Cl2/(Cl2 + CO) from 0.125 to 0.857 does not modify the carbochlorination mechanism of Cr2O3. In these conditions, the reaction rate passes through a maximum when using a chlorinating gas mixture having a Cl2/(Cl2 + CO) ratio of about 0.5.
Deoxidation of Molten Copper with a Rotating Graphite Cylinder
MASAKI MIYATA, MASAMICHI SANO, and MASAHIRO HIRASAWA
The kinetics of deoxidation of molten copper by the "vacuum-suction degassing" (VSD) method is investigated. The molten copper is deoxidized by a rotating porous graphite tube immersed in the copper bath. The inside space of the porous graphite tube is evacuated so that the CO gas formed at the graphite-metal interface is removed through the tube wall. The experimental results suggest that the mass transfer of oxygen in the metal phase controls the reaction rate. The kinetic data are arranged with a first-order rate equation. At (ppm O)  10, the rate constant increases by decreasing the porosity of the graphite and increasing the thickness of the tube wall. This result suggests that the suction of CO gas weakens CO bubble stirring and, thereby, the mass transfer at the tube-melt interface. However, when the rate of CO suction becomes comparable to or larger than the CO gas evolution rate, the effect of CO stirring becomes negligible. This situation appears under the conditions of high porosity and large wall thickness at (ppm O)
10, the rate constant increases by decreasing the porosity of the graphite and increasing the thickness of the tube wall. This result suggests that the suction of CO gas weakens CO bubble stirring and, thereby, the mass transfer at the tube-melt interface. However, when the rate of CO suction becomes comparable to or larger than the CO gas evolution rate, the effect of CO stirring becomes negligible. This situation appears under the conditions of high porosity and large wall thickness at (ppm O)  10. At the low oxygen concentration range of (ppm O)
10. At the low oxygen concentration range of (ppm O) 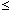 4, the effect of CO stirring becomes negligible, regardless of the CO suction condition, because of the considerably low CO formation rate. The achievement of deoxidation by the VSD method is evaluated in connection with the final oxygen concentration.
4, the effect of CO stirring becomes negligible, regardless of the CO suction condition, because of the considerably low CO formation rate. The achievement of deoxidation by the VSD method is evaluated in connection with the final oxygen concentration.
Communication: Reduction of FeO in Smelting Slags by Solid Carbon: Re-Examination of the Influence of the Gas-Carbon Reaction
S.R. STORY, B. SARMA, R.J. FRUEHAN, A.W. CRAMB, and G.R. BELTON
ELECTROMETALLURGY
Electrochemical Characterization of Copper Deposited on Plasma and Thermally Modified Titanium Surfaces
K.S. TENG, J.-L. DELPLANCKE, J. ZHANG, and T.J. O'KEEFE
Thin oxide films were grown at temperatures from 373 to 1073 K in plasma and in air on commercially pure titanium substrates. It was determined that the color, thickness, composition, phase, and polarization behavior in a copper electrolyte varied with operating conditions: temperature, oxygen partial pressure, and plasma composition. High-temperature and high oxygen partial pressure plasma produced a thick oxide film. The surface film structure transformed from TiO2 (anatase) to TiO2 (rutile) at a temperature of 600°C. A lower oxide of the form TinO2n-1, such as Ti2O3 (which may be porous) or possibly Ti3O5, was formed on a thermally treated sample (400°C, 80 mtorr O2, 3 hours). This sample exhibited the lowest potential for copper nucleation and gave a very uniform, smooth, and hole-free copper foil.
TRANSPORT PHENOMENA
Water Model Study of the Frequency of Bubble Formation under Reduced and Elevated Pressures
MANABU IGUCHI and TOMOYUKI CHIHARA
Air was injected vertically upward into a water bath through a bottom nozzle or a bottom orifice. The surface pressure was reduced or elevated from an atmospheric pressure in order to change the hydrostatic pressure around the nozzle and orifice. The gas delivery system was designed so that bubbles were generated in the middle and high gas flow rate regimes under a constant flow condition. The frequency of bubble formation, fB, decreased as the surface pressure, Ps, decreased when the volumetric gas flow rate, Qg, was kept constant. The measured fB values were predicted satisfactorily by an empirical equation proposed previously by the present authors. This equation was derived originally to correlate the frequency of bubble formation both in aqueous and molten metal systems under an atmospheric surface pressure. The effect of surface pressure on the frequency of bubble formation was considered in terms of the density of gas,  g, and the volumetric gas flow rate Qg in the aforementioned empirical equation. These two quantities,
g, and the volumetric gas flow rate Qg in the aforementioned empirical equation. These two quantities,  g and Qg, were evaluated at the nozzle exit by using the hydrostatic pressure there.
g and Qg, were evaluated at the nozzle exit by using the hydrostatic pressure there.
Interdiffusivities and Mass Transfer Coefficients of NaF Gas
Y. KASHIWAYA and A.W. CRAMB
To clarify the vaporization phenomena of sodium fluoride (NaF), the interdiffusivities of NaF in N2, Ar, and He were measured by thermogravimetric analysis from 1300 to 1770 K. At 1540 K, the interdiffusivity of NaF in N2 (2.46 cm2/s) was slightly larger than that in Ar (2.40 cm2/s), while the interdiffusivity of NaF in He (6.00 cm2/s) was significantly larger than those in the N2 and Ar systems. The Chapman Enskog equation was used to calculate the interdiffusivities of NaF in N2, Ar, and He. Calculated results were in good agreement with observations for nitrogen and argon; however, calculated results for the NaF-He system were significantly higher than those observed. Overall experimental mass transfer coefficients were described by a Ranz
Enskog equation was used to calculate the interdiffusivities of NaF in N2, Ar, and He. Calculated results were in good agreement with observations for nitrogen and argon; however, calculated results for the NaF-He system were significantly higher than those observed. Overall experimental mass transfer coefficients were described by a Ranz Marshall type correlation based upon the Nusselt number (Nu), the Reynolds number (Re), and the Schmidt number (Sc):
Marshall type correlation based upon the Nusselt number (Nu), the Reynolds number (Re), and the Schmidt number (Sc):
Nu = 7.0 + 0.28 (Re)4(Sc)2
in which the viscosities in the NaF-N2, Ar, or He system were calculated from the Lennard Jones parameters developed in this study.
Jones parameters developed in this study.
Modeling Studies of Fluid Flow below Flash-Smelting Burners Including Transient Behavior
I.D. SUTALO, J.A. HARRIS, F.R.A. JORGENSEN, and N.B. GRAY
Water-model experiments were carried out on 1:14-scale models of venturi, distributor, and jet-flow burners to ascertain flow patterns at varying Reynolds numbers (60,000 to 507,000) using time-lapse streak photography and video streak photography. Digital particle image velocimetry (DPIV) was used to determine the axial and radial velocities and to estimate the turbulence kinetic-energy field beneath the distributor burner. In the DPIV experiments, a temporal instability in the main jet exiting from the burner occurred at a Reynolds number = 104,000, a Strouhal number  3 X 10-3, and a large expansion ratio (shaft/burner-diameter ratio = 10). The main jet usually pointed away from the burner inlet but was also observed to fluctuate and precess in a quasi-random fashion. Recommendations are made for improving flash-smelting burner performance by promoting conditions to eliminate precessing. The use of higher Reynolds numbers was recommended to improve both the use of shaft volume and the mixing of the concentrate particles and gas stream. A three-dimensional (3-D) mathematical model was used to simulate the water flow through the distributor burner, shaft, and settler. The predicted velocity field consisted of a main jet pointing away from the burner inlet and a large recirculation zone in the center of the shaft. The predicted and measured velocity magnitudes compared well in the recirculation zone, but the steady-state mathematical model predicted higher velocity values in the main jet than were experimentally determined.
3 X 10-3, and a large expansion ratio (shaft/burner-diameter ratio = 10). The main jet usually pointed away from the burner inlet but was also observed to fluctuate and precess in a quasi-random fashion. Recommendations are made for improving flash-smelting burner performance by promoting conditions to eliminate precessing. The use of higher Reynolds numbers was recommended to improve both the use of shaft volume and the mixing of the concentrate particles and gas stream. A three-dimensional (3-D) mathematical model was used to simulate the water flow through the distributor burner, shaft, and settler. The predicted velocity field consisted of a main jet pointing away from the burner inlet and a large recirculation zone in the center of the shaft. The predicted and measured velocity magnitudes compared well in the recirculation zone, but the steady-state mathematical model predicted higher velocity values in the main jet than were experimentally determined.
Monte Carlo Simulation of Clustering of Alumina Particles in Turbulent Liquid Aluminum
C. TIAN, G.A. IRONS, and D.S. WILKINSON
A Monte Carlo method was developed to simulate the formation of clusters of alumina particles suspended in a turbulent pure liquid aluminum melt. With this approach, the chaotic movements of small alumina particles suspended within eddies smaller than the Kolmogoroff microscale were treated in a similar way to Brownian motion, and clusters were assumed to form once these particles collided with each other. The results obtained from the simulation indicate that clusters form very quickly during vigorous stirring and that the formation kinetics at the very beginning of mixing follow a second-order behavior. Clustering has been observed previously in the SiC-Al system and was also observed in the Al2O3-Al system in the present work.
PROCESS CONTROL
Characterization of Spray Atomization of 3003 Aluminum Alloy during Linear Spray Atomization and Deposition
YIZHANG ZHOU, STEVEN LEE, VINCENT G. McDONELL, SCOTT SAMUELSEN, ROBERT L. KOZAREK, and ENRIQUE J. LAVERNIA
Linear spray atomization and deposition is an attractive technique to produce near-net-shape deposits, such as aluminum sheet and strip. In the present study, phase Doppler interferometry (PDI) was used in a backscatter mode to characterize, in situ, the droplet size and velocity distributions during linear spray atomization and deposition of a 3003 aluminum alloy. The PDI measurements were obtained along axes corresponding to the direction parallel to the nozzle slit and to the direction perpendicular to the slit. The PDI results delineate the temporal and spatial distribution of the droplet size and velocity during the metal spray. Both point and ``line'' measurements were obtained and are reported. The line measurements resulted from the integration of measurement made along a line scan obtained in real time (i.e., not ensemble averaged). Postrun analysis of the droplet size distribution using laser diffraction and sieving techniques is also reported. The PDI point measurements revealed that droplet size and velocity distribution were relatively invariant with time. The line measurements of droplet velocity showed that the droplet axial velocity exhibits a bimodal behavior, which becomes more apparent with increasing atomizing gas pressure, a result of droplet recirculation inside the spray chamber. In addition, the peak in the droplet axial velocity distribution increased as atomizing gas pressure increased. The line characterization also showed that the droplet size distribution became more homogeneous with increasing gas pressure, and that the distribution characteristic diameters of droplets decreased consistently with increasing gas pressure. Postrun characterization of the droplet size distribution of the entire metal spray using diffraction and sieving methods indicated that the mass (volume) median diameter D50 and the Sauter mean diameter (SMD) D32 decreased with increasing gas pressure in a manner consistent with PDI results.
PHYSICAL CHEMISTRY
Thermochemistry of Ternary Liquid Cu-Mg-Si Alloys
V. GANESAN, HARALD FEUFEL, FERDINAND SOMMER, and HERBERT IPSER
The thermodynamic properties of ternary liquid Cu-Mg-Si alloys with a constant concentration ratio of xCu/xSi = 7/3 were determined using a combination of different experimental methods. Enthalpies of mixing were measured by isoperibolic calorimetry, and magnesium vapor pressures were obtained by an isopiestic method. Partial thermodynamic properties of magnesium were derived from the vapor pressure data, and the composition dependence of the magnesium activities is given for 1173 K. Integral Gibbs energies of mixing for liquid alloys were calculated by a Gibbs Duhem integration. The agreement of the thermodynamic data obtained from different methods was found to be very good.
Duhem integration. The agreement of the thermodynamic data obtained from different methods was found to be very good.
Standard Enthalpies of Formation for some Samarium Alloys, Sm + Me (Me = Ni, Rh, Pd, Pt), Determined by High-Temperature Direct Synthesis Calorimetry
QITI GUO and OLE J. KLEPPA
The standard enthalpies of formation of eight samarium alloys with late transition metals have been determined by direct synthesis calorimetry at 1273 ± 2 K. The following values of  H0f, in kJ·(mole atom)-1, are reported: SmNi5, -27.4 ± 0.5; Sm5Rh4, -66.5 ± 1.0; SmRh2, -65.5 ± 1.2; SmPd, -82.4 ± 2.0; Sm3Pd4, -87.2 ± 2.5; SmPd3, -82.9 ± 2.5; SmPt, -108.7 ± 3.5; and SmPt2, -100.2 ± 2.6. The results are compared with predicted values from the Miedema model, with available literature data for SmNi5, SmPd, and SmPt, and with earlier values for similar compounds formed by other lanthanide metals reported by this laboratory. The observed relationships between the enthalpies of formation and the number of f-electrons in the considered binary alloys REnMem (RE = lanthanide elements; and Me = Group VIII elements) are discussed.
H0f, in kJ·(mole atom)-1, are reported: SmNi5, -27.4 ± 0.5; Sm5Rh4, -66.5 ± 1.0; SmRh2, -65.5 ± 1.2; SmPd, -82.4 ± 2.0; Sm3Pd4, -87.2 ± 2.5; SmPd3, -82.9 ± 2.5; SmPt, -108.7 ± 3.5; and SmPt2, -100.2 ± 2.6. The results are compared with predicted values from the Miedema model, with available literature data for SmNi5, SmPd, and SmPt, and with earlier values for similar compounds formed by other lanthanide metals reported by this laboratory. The observed relationships between the enthalpies of formation and the number of f-electrons in the considered binary alloys REnMem (RE = lanthanide elements; and Me = Group VIII elements) are discussed.
Interfacial Tension between Aluminum and NaCl-KCl Based Salt Systems
Based Salt Systems
RAJA R. ROY and TORSTEIN A. UTIGARD
Aluminum scrap is frequently remelted under a NaCl-KCl based salt flux cover to prevent oxidation, to aid in the stripping of oxide films, and to improve drop coalescence. In this process, the interfacial tension between the aluminum metal and the salt flux plays an important role. However, the measurement of interfacial tensions at high temperature is difficult and prone to errors. Therefore, an interfacial tension model, presented in this article, has been developed. The interfacial tension between aluminum and NaCl-KCl based melts does not change with the addition of chlorides or with variations in the composition of the NaCl-KCl melt. On the other hand, the addition of fluorides decreases the interfacial tension to various extents due to the adsorption of sodium and/or potassium at the interface. Addition of AlF3 is the least effective; additions of LiF, MgF2, CaF2, BaF2, or SrF2 are moderately effective; and additions of NaF or KF are the most effective in lowering the interfacial tension.
Steady-State Studies of the Reactions of H2O-CO and CO2-H2 Mixtures with Liquid Iron
Y. SASAKI and G.R. BELTON
Studies have been made of the steady-state composition of liquid iron exposed to high flow rates of H2O-CO mixtures at 1550°C to 1700°C and CO2-H2 mixtures at 1600°C. Values of the steady-state activity of oxygen have been established by measurement of either the carbon concentration or the silicon concentration when the iron was held in a silica crucible. Additions of sulfur or selenium to the iron have been found to result in steady-state oxygen activities, which differ significantly from those expected from water-gas equilibrium. The results are interpreted to show that the ratio of the apparent first-order rate constants for the reactions of H2O and CO2 with liquid iron is about 3 at 1600°C. It is shown that the dependencies of the rate constants on the activities of sulfur, oxygen, and selenium must, even if complex, be similar for the H2O and CO2 reactions with liquid iron, to a good approximation.
Iron Redox Equilibria in CaO-Al2O3-SiO2 and MgO-CaO-Al2O3-SiO2 Slags
LIXIANG YANG and G.R. BELTON
Measurements have been made of the ratio of ferric to ferrous iron in CaO-Al2O3-SiO2 and MgO-CaO-Al2O3-SiO2 slags at oxygen activities ranging from equilibrium with pCO2/pCO  0.01 to as high as air at temperatures of 1573 to 1773 K. At 1773 K, values are given by
0.01 to as high as air at temperatures of 1573 to 1773 K. At 1773 K, values are given by
log  = 0.3(±0.02) Y + 0.45(±0.01) log
= 0.3(±0.02) Y + 0.45(±0.01) log
 - 1.24(±0.01)
- 1.24(±0.01)
where Y = (CaO + MgO)/SiO2, for melts with the molar ratio of CaO/SiO2 = 0.45 to 1.52, 10 to 15 mol pct Al2O3, up to 12 mol pct MgO (at CaO/SiO2  1.5), and with 3 to 10 wt pct total Fe. Available evidence suggests that, to a good approximation, these redox equilibria are independent of temperature when expressed with respect to pCO2/pCO, probably from about 1573 to 1873 K. Limited studies have also been carried out on melts containing about 40 mol pct Al2O3, up to 12 mol pct MgO (at CaO/SiO2
1.5), and with 3 to 10 wt pct total Fe. Available evidence suggests that, to a good approximation, these redox equilibria are independent of temperature when expressed with respect to pCO2/pCO, probably from about 1573 to 1873 K. Limited studies have also been carried out on melts containing about 40 mol pct Al2O3, up to 12 mol pct MgO (at CaO/SiO2  1.5), and 3.6 to 4.7 wt pct Fe. These show a strongly nonideal behavior for the iron redox equilibrium, with
1.5), and 3.6 to 4.7 wt pct Fe. These show a strongly nonideal behavior for the iron redox equilibrium, with


 0.37
0.37
The nonideal behavior and the effects of basicity and Al2O3 concentration on the redox equilibria are discussed in terms of the charge balance model of alumino-silicates and the published structural information from Mössbauer and NMR (Nuclear Magnetic Resonance) spectroscopy of quenched melts.
SOLIDIFICATION
Modeling Freckle Formation in Three Dimensions during Solidification of Multicomponent Alloys
S.D. FELICELLI, D.R. POIRIER, and J.C. HEINRICH
The formation of macrosegregation defects known as "freckles" was simulated using a three-dimensional finite element model that calculates the thermosolutal convection and macrosegregation during the dendritic solidification of multicomponent alloys. A recently introduced algorithm was used to calculate the complicated solidification path of alloys of many components, which can accommodate liquidus temperatures that are general functions of liquid concentrations. The calculations are started from an all-liquid state, and the growth of the mushy zone is followed in time. Simulations of a Ni-Al-Ta-W alloy were performed on a rectangular cylinder until complete solidification. The results reveal details of the formation of freckles not previously observed in two-dimensional simulations. Liquid plumes in the form of chimney convection emanate from channels within the mushy zone, with similar qualitative features previously observed in transparent systems. Associated with the formation of channels, there is a complex three-dimensional flow produced by the interaction of the different solutal buoyancies of the alloy solutes. Regions of enhanced solid growth develop around the channel mouths, which are visualized as volcanoes on top of the mushy zone. The prediction of volcanoes differs from our previous calculations with multicomponent alloys in two dimensions, in which the volcanoes were not nearly as apparent. These and other features of freckle formation phenomena are illustrated.
MATERIALS PROCESSING
Microstructural Effects on Distortion and Solid-Liquid Segregation during Liquid Phase Sintering under Microgravity Conditions
JOHN L. JOHNSON, A. UPADHYAYA and RANDALL M. GERMAN
Tungsten heavy alloys with compositions ranging from 78 to 98 wt pct tungsten were liquid phase sintered at 1507°C under microgravity conditions for 120 minutes. The sintered microstructures were quantitatively measured for solid volume fraction, grain size, connectivity, and contiguity. Links between these microstructural parameters were analyzed and compared to previously derived empirical equations. The macrostructures of the samples were also quantified and correlated to the underlying microstructures. Critical values of solid volume fraction, contiguity, and connectivity required for free-standing structural rigidity were defined for various degrees of bond rigidity as represented by the dihedral angle. The results are used to predict the degree of solid-liquid segregation due to density differences between the solid and the liquid.
Combustion Characteristics of the Ni3Ti-TiB2 Intermetallic Matrix Composites
H.C. YI, T.C. WOODGER, J.Y. GUIGN;aaE, and J.J. MOORE
Combustion synthesis (SHS) of Ni3Ti-TiB2 metal matrix composites (MMCs) was selected to investigate the effect of gravity in a reaction system that produced a light, solid ceramic particle (TiB2) synthesized in situ in a large volume (>50 pct) of the liquid metallic matrix (Ni3Ti). The effects of composition, green density of pellets, and nickel particle size on the combustion characteristics are presented. Combustion reaction temperature, wave velocity, and combustion behavior changed drastically with change in reaction parameters. Two types of density effects were observed when different nickel particle sizes were used. The structures of the combustion zones were characterized using temperature profile analysis. The combustion zone can be divided into preflame, reaction, and afterburning zones. The combustion mechanism was studied by quenching the combustion front. It was found that the combustion reactions proceeded in the following sequence: formation of liquid Ni-Ti eutectic at 940°C 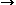 Ni3Ti + NiTi phases
Ni3Ti + NiTi phases 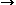 reduction of NiTi with B
reduction of NiTi with B 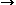 TiB2 + Ni3Ti.
TiB2 + Ni3Ti.
Combustion Synthesis of HfB2-Al Composites
H.C. YI, T.C. WOODGER, J.J. MOORE, and J.Y. GUIGNÉ
Combustion synthesis (SHS) of HfB2-Al composite materials with a wide range of HfB2-to-Al ratios corresponding to either metal (Al) or ceramic (HfB2) matrix was carried out with the emphasis on 60 and 70 vol pct Al. The effects of composition and green density of pellets on the combustion characteristics were studied. Combustion temperature, wave velocity, and reaction mode all changed drastically with composition and green density. The combustion mechanisms were also studied using temperature profile analysis. The combustion zone can be divided into preflame and main reaction zones, and the width of the latter was much larger than that of the former. It was also found that the combustion reaction was initiated at the melting of the aluminum and consisted of a two-step reaction sequence corresponding to the initial formation of Al3Hf and, subsequently, HfB2 compounds. The formation of Al3Hf triggered the HfB2 formation according to the following reaction mechanism:
Al3Hf + 2B 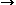 HfB2 + 3Al
HfB2 + 3Al
The Effect of Gravity on the Combustion Synthesis of Metal-Ceramic Composites
H.C. YI, T.C. WOODGER, J.J. MOORE, and J.Y. GUIGN;aaE
The effects of gravity on the combustion characteristics and microstructure of metal-ceramic composites (HfB2/Al and Ni3Ti/TiB2 systems) were studied under both normal and low gravity conditions. Under normal gravity conditions, pellets were ignited in three orientations relative to the gravity vector. Low gravity combustion synthesis (SHS) was carried out on a DC-9 aircraft at the NASA Lewis Research Center. It was found that under normal gravity conditions, both the combustion temperature and wave velocity were highest when the pellet was ignited from the bottom orientation; i.e., the wave propagation direction was directly opposed to the gravitational force. The SHS of 70 vol pct Al (in the Al-HfB2 system) was changed from unstable, slow, and incomplete when ignited from the top to unstable, faster, and complete combustion when ignited from the bottom. The hydrostatic force (height X density X gravity) in the liquid aluminum was thought to be the cause of formation of aluminum nodules at the surface of the pellet. The aluminum nodules that were observed on the surface of the pellet when reacted under normal gravity were totally absent for reactions conducted under low gravity. Buoyancy of the TiB2 particles and sedimentation of the Ni3Ti phase were observed for the Ni3Ti/TiB2 system. The possibility of liquid convective flow at the combustion front was also discussed. Under low gravity conditions, both the combustion temperature and wave velocity were lower than those under normal gravity. The distribution of the ceramic phase, i.e., TiB2 or HfB2, in the intermetallic (Ni3Ti) or reactive (Al) matrix was more uniform.
Lewis Research Center. It was found that under normal gravity conditions, both the combustion temperature and wave velocity were highest when the pellet was ignited from the bottom orientation; i.e., the wave propagation direction was directly opposed to the gravitational force. The SHS of 70 vol pct Al (in the Al-HfB2 system) was changed from unstable, slow, and incomplete when ignited from the top to unstable, faster, and complete combustion when ignited from the bottom. The hydrostatic force (height X density X gravity) in the liquid aluminum was thought to be the cause of formation of aluminum nodules at the surface of the pellet. The aluminum nodules that were observed on the surface of the pellet when reacted under normal gravity were totally absent for reactions conducted under low gravity. Buoyancy of the TiB2 particles and sedimentation of the Ni3Ti phase were observed for the Ni3Ti/TiB2 system. The possibility of liquid convective flow at the combustion front was also discussed. Under low gravity conditions, both the combustion temperature and wave velocity were lower than those under normal gravity. The distribution of the ceramic phase, i.e., TiB2 or HfB2, in the intermetallic (Ni3Ti) or reactive (Al) matrix was more uniform.
WELDING & JOINING
Communication: On Optimization of the Powder Plasma Arc Surfacing Process
J.N. DUPONT
MATHEMATICAL MODELING
Bioleaching Model of a Copper-Sulfide Ore Bed in Heap and Dump Configurations
J.M. CASAS, J. MARTINEZ, L. MORENO, and T. VARGAS
A two-dimensional (2-D) model for a heap or dump bioleaching of a copper ore containing mainly chalcocite and pyrite has been developed. The rate of the mineral sulfide dissolution was related to the rate of oxidation by bacteria attached onto the ore surface. The latter was calculated using the model of Michaelis Menten, where both temperature and dissolved oxygen in the leach solution were taken into account by the kinetic equation. Oxygen transport through the ore bed was associated with natural air convection originating from the decrease in gas density inside the ore bed, which was attributable not only to heating, but also to humidification and decrease in the oxygen concentration. The model was used to estimate air-velocity fields and profiles of temperature and oxygen concentrations as well as mineral conversions during the bioleaching operation for ore beds with different pyrite contents, bacterial populations, widths, heights, and permeabilities. The model provides a useful tool for the design, improvement, and optimization of industrial operating conditions.
Menten, where both temperature and dissolved oxygen in the leach solution were taken into account by the kinetic equation. Oxygen transport through the ore bed was associated with natural air convection originating from the decrease in gas density inside the ore bed, which was attributable not only to heating, but also to humidification and decrease in the oxygen concentration. The model was used to estimate air-velocity fields and profiles of temperature and oxygen concentrations as well as mineral conversions during the bioleaching operation for ore beds with different pyrite contents, bacterial populations, widths, heights, and permeabilities. The model provides a useful tool for the design, improvement, and optimization of industrial operating conditions.
Numerical and Experimental Study of a Hydrodynamic Cavitation Tube
H. HU, Z. ZHOU, Z. XU, and J.A. FINCH
A numerical analysis of hydrodynamics in a cavitation tube used for activating fine particle flotation is described. Using numerical procedures developed for solving the turbulent k- model with boundary fitted coordinates, the stream function, vorticity, velocity, and pressure distributions in a cavitation tube were calculated. The calculated pressure distribution was found to be in excellent agreement with experimental results. The requirement of a pressure drop below approximately 10 m water for cavitation to occur was observed experimentally and confirmed by the model. The use of the numerical procedures for cavitation tube design is discussed briefly.
model with boundary fitted coordinates, the stream function, vorticity, velocity, and pressure distributions in a cavitation tube were calculated. The calculated pressure distribution was found to be in excellent agreement with experimental results. The requirement of a pressure drop below approximately 10 m water for cavitation to occur was observed experimentally and confirmed by the model. The use of the numerical procedures for cavitation tube design is discussed briefly.
A Mathematical Model for the Dynamic Behavior of Melts Subjected to Electromagnetic Forces: Part I. Model Development and Comparison of Predictions with Published Experimental Results
R. KAGEYAMA and J.W. EVANS
A mathematical model was developed to predict electromagnetically driven flow and (particularly) free surface behavior in melts subject to electromagnetic forces. Such melts appear in electromagnetic casters, induction furnaces, and other metal processing units. The calculations started with Maxwell's equations and Ohm's law, which were solved by a novel "modified hybrid technique." The instantaneous continuity and Navier Stokes equations (rather than their time-averaged versions) were then solved with electromagrretic forces as input. The calculations allowed for the dynamic behavior of the free surface of the melt, and electromagnetic fields were recomputed as the free surface changed. In this first part of a two-part article, the model predictions are compared with the experimental measurements of induced current, magnetic field, melt velocity, and free surface deformation reported by others.
Stokes equations (rather than their time-averaged versions) were then solved with electromagrretic forces as input. The calculations allowed for the dynamic behavior of the free surface of the melt, and electromagnetic fields were recomputed as the free surface changed. In this first part of a two-part article, the model predictions are compared with the experimental measurements of induced current, magnetic field, melt velocity, and free surface deformation reported by others.
Communication: Decay of Fluid Motion in a Filling Ladle after Tapping
G.G. ROY
Direct questions about this or any other Metallurgical and Materials Transactions page to mettrans@andrew.cmu.edu.

 10, the rate constant increases by decreasing the porosity of the graphite and increasing the thickness of the tube wall. This result suggests that the suction of CO gas weakens CO bubble stirring and, thereby, the mass transfer at the tube-melt interface. However, when the rate of CO suction becomes comparable to or larger than the CO gas evolution rate, the effect of CO stirring becomes negligible. This situation appears under the conditions of high porosity and large wall thickness at (ppm O)
10, the rate constant increases by decreasing the porosity of the graphite and increasing the thickness of the tube wall. This result suggests that the suction of CO gas weakens CO bubble stirring and, thereby, the mass transfer at the tube-melt interface. However, when the rate of CO suction becomes comparable to or larger than the CO gas evolution rate, the effect of CO stirring becomes negligible. This situation appears under the conditions of high porosity and large wall thickness at (ppm O) 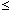 4, the effect of CO stirring becomes negligible, regardless of the CO suction condition, because of the considerably low CO formation rate. The achievement of deoxidation by the VSD method is evaluated in connection with the final oxygen concentration.
4, the effect of CO stirring becomes negligible, regardless of the CO suction condition, because of the considerably low CO formation rate. The achievement of deoxidation by the VSD method is evaluated in connection with the final oxygen concentration.
 g, and the volumetric gas flow rate Qg in the aforementioned empirical equation. These two quantities,
g, and the volumetric gas flow rate Qg in the aforementioned empirical equation. These two quantities,  Enskog equation was used to calculate the interdiffusivities of NaF in N2, Ar, and He. Calculated results were in good agreement with observations for nitrogen and argon; however, calculated results for the NaF-He system were significantly higher than those observed. Overall experimental mass transfer coefficients were described by a Ranz
Enskog equation was used to calculate the interdiffusivities of NaF in N2, Ar, and He. Calculated results were in good agreement with observations for nitrogen and argon; however, calculated results for the NaF-He system were significantly higher than those observed. Overall experimental mass transfer coefficients were described by a Ranz 3 X 10-3, and a large expansion ratio (shaft/burner-diameter ratio = 10). The main jet usually pointed away from the burner inlet but was also observed to fluctuate and precess in a quasi-random fashion. Recommendations are made for improving flash-smelting burner performance by promoting conditions to eliminate precessing. The use of higher Reynolds numbers was recommended to improve both the use of shaft volume and the mixing of the concentrate particles and gas stream. A three-dimensional (3-D) mathematical model was used to simulate the water flow through the distributor burner, shaft, and settler. The predicted velocity field consisted of a main jet pointing away from the burner inlet and a large recirculation zone in the center of the shaft. The predicted and measured velocity magnitudes compared well in the recirculation zone, but the steady-state mathematical model predicted higher velocity values in the main jet than were experimentally determined.
3 X 10-3, and a large expansion ratio (shaft/burner-diameter ratio = 10). The main jet usually pointed away from the burner inlet but was also observed to fluctuate and precess in a quasi-random fashion. Recommendations are made for improving flash-smelting burner performance by promoting conditions to eliminate precessing. The use of higher Reynolds numbers was recommended to improve both the use of shaft volume and the mixing of the concentrate particles and gas stream. A three-dimensional (3-D) mathematical model was used to simulate the water flow through the distributor burner, shaft, and settler. The predicted velocity field consisted of a main jet pointing away from the burner inlet and a large recirculation zone in the center of the shaft. The predicted and measured velocity magnitudes compared well in the recirculation zone, but the steady-state mathematical model predicted higher velocity values in the main jet than were experimentally determined.
 H0f, in kJ·(mole atom)-1, are reported: SmNi5, -27.4 ± 0.5; Sm5Rh4, -66.5 ± 1.0; SmRh2, -65.5 ± 1.2; SmPd, -82.4 ± 2.0; Sm3Pd4, -87.2 ± 2.5; SmPd3, -82.9 ± 2.5; SmPt, -108.7 ± 3.5; and SmPt2, -100.2 ± 2.6. The results are compared with predicted values from the Miedema model, with available literature data for SmNi5, SmPd, and SmPt, and with earlier values for similar compounds formed by other lanthanide metals reported by this laboratory. The observed relationships between the enthalpies of formation and the number of f-electrons in the considered binary alloys REnMem (RE = lanthanide elements; and Me = Group VIII elements) are discussed.
H0f, in kJ·(mole atom)-1, are reported: SmNi5, -27.4 ± 0.5; Sm5Rh4, -66.5 ± 1.0; SmRh2, -65.5 ± 1.2; SmPd, -82.4 ± 2.0; Sm3Pd4, -87.2 ± 2.5; SmPd3, -82.9 ± 2.5; SmPt, -108.7 ± 3.5; and SmPt2, -100.2 ± 2.6. The results are compared with predicted values from the Miedema model, with available literature data for SmNi5, SmPd, and SmPt, and with earlier values for similar compounds formed by other lanthanide metals reported by this laboratory. The observed relationships between the enthalpies of formation and the number of f-electrons in the considered binary alloys REnMem (RE = lanthanide elements; and Me = Group VIII elements) are discussed.
 = 0.3(±0.02) Y + 0.45(±0.01) log
= 0.3(±0.02) Y + 0.45(±0.01) log - 1.24(±0.01)
- 1.24(±0.01)

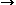 Ni3Ti + NiTi phases
Ni3Ti + NiTi phases  model with boundary fitted coordinates, the stream function, vorticity, velocity, and pressure distributions in a cavitation tube were calculated. The calculated pressure distribution was found to be in excellent agreement with experimental results. The requirement of a pressure drop below approximately 10 m water for cavitation to occur was observed experimentally and confirmed by the model. The use of the numerical procedures for cavitation tube design is discussed briefly.
model with boundary fitted coordinates, the stream function, vorticity, velocity, and pressure distributions in a cavitation tube were calculated. The calculated pressure distribution was found to be in excellent agreement with experimental results. The requirement of a pressure drop below approximately 10 m water for cavitation to occur was observed experimentally and confirmed by the model. The use of the numerical procedures for cavitation tube design is discussed briefly.