 |
|
|---|
 |
|
|---|
 |
|---|
| TABLE OF CONTENTS |
Both announcements were historically significant. NSC, with partner Mitsubishi Heavy Industries (MHI), announced that their highly secret strip-casting project at the Hikari works was producing significant quantities (20,000 tonnes per month) of commercial-quality stainless steels. BHP and their partner IHI announced completion of their even more secret development of a similar scale project for low -carbon steels (Figure 1). Thus, by year end, it was clear that it was technologically possible to direct-cast 60 tonne heats of liquid steel and form coiled steel strip that is 1–3 mm thick, up to 1.345 m wide, and cast at speeds in excess of 60 m/min. In addition, it was shown that it is possible to develop a continuous-casting process for steel that produces a strip that is geometrically similar to a hot-rolled steel strip product. Both developers project that a commercial plant would be capable of producing between 300,000 tonnes and 500,000 tonnes per year.
Both casting technologies—the oscillating and the travelling mold, are based upon two completely separate technologies that result in the formation of the surface of the casting. The solidifying shell is formed by heat transfer through a molten oxide layer that separates the solidifying shell from the mold surface in the oscillating mold processes. In the twin-roll caster there is direct contact between the shell and the mold surface. Thus, shell formation and growth in oscillating casters is controlled by the physical characteristics of the oxide flux, while in the twin-roll caster it is controlled by contact resistance between the shell and the mold surface. Clearly, mold-heat fluxes are significantly higher in twin-roll casters (10–20 MW/m2) than in oscillating mold casters (1–5 MW/m2), and this results in significant differences in cast structure between the two processes.
 |
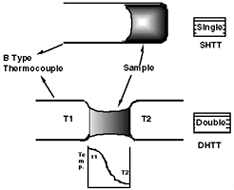 |
| Figure 1. Examples of twin-roll, cast, low-carbon steel coils cast by BHP/IHI, at Project M, Port Kembla, New South Wales. (Figure courtesy of BHP.) | Figure 2. A double- and single-hot thermocouple. |
The parameters controlling solidification in casting processes are specific to the details of the process. This has led to the development of a number of experimental techniques that are aimed at simulating actual casting environments. Three of these techniques are
 |
 |
| a | b |
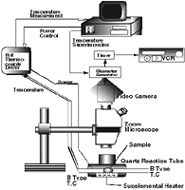
| Figure 3. (a) A schematic of the DHTT and (b) the experimental setup. | |
The mold slags are liquid oxides and easy glass formers under high cooling rates. Thus, it is necessary to describe the conditions under which glass formation is possible, the conditions for the initiation of solidification, the crystal morphology, chemistry and growth rate, and the time evolution of the fraction of solid. It is well known that the onset of crystallization in liquid slags must be a function of cooling rate and that to determine the solidification behavior of a liquid slag one must construct either isothermal time temperature transformation diagrams (TTT curves) or continuous cooling transformation diagrams (CCT curves). In addition, the growth rate, morphology, and solidified fraction of the slag under varying cooling rates are important in the determination of the effect of crystallization of the slag on heat transfer and rheology. Thus, a technique whereby the thermal field can be determined as the solidification process is observed was developed. This technique, the double hot thermocouple technique, combines the hot thermocouple technique with video observation and image analysis, allowing crystal-growth rates, morphologies, and solidified fractions to be determined under defined thermal conditions.
The concept of the single hot thermocouple technique dates back to the end of 19th century, and the hot thermocouple method itself was successfully developed by Ordway4 and Welch et al.5 in the 1950s. The progress of electronic development has made the hot thermocouple method easier and more reliable and has led to renewed interest in the technique.6 Ohta et al.7 applied the hot thermocouple method to the measurement of liquidus temperatures, the clarification of the existence of two phase regions in slags, and to the understanding of slag reactions. More recently (1993), Asayama et al.8 discussed glass formation in silicate slags using the hot thermocouple method.
 |
 |
Due to the low mass of the system (sample and thermocouple), high heating and cooling rates can be easily obtained. This is very useful for determination of TTT and CCT diagrams where fast cooling rates are required.
The experimental apparatus of the DHTT consists of two systems: an observation system and a thermocouple system. The observation system includes a microscope with a three charge couple device color video camera connected to a videocassette recorder. The thermocouple system includes the two thermocouples located in a reaction tube below the microscope, and the sample is placed between the thermocouples. Each thermocouple is connected to a separate thermocouple controller, which is a unique system to enable simultaneous measurement of temperature while heating a thermocouple. A computer controls the two thermocouple controllers making it possible to individually control heating and cooling conditions of the thermocouples. Figure 3 shows a schematic of the experimental apparatus and a photograph of the setup.
 |
 |
 |
| a | b | c |
 |
 |
 |
| d | e | f |
| Figure 6. The fragmentation process at (a) t = 161 s, (b) t = 167 s, (c) t = 173 s, (d) t = 179 s, (e) t = 189 s, and (f) 199 s. | ||
The effect of fluid flow also has a striking effect on the crystal stability. For example, it is often seen that growing crystals are not stable and break apart under the stresses imparted by the flow under conditions where a high rate of internal recirculation is observed. An example of this phenomenon can be seen in the video clip of Figure 5 and the photographs in Figure 6, where a growing equiaxed dendrite of calcium aluminate can be seen to fragment under the action of a shear flow that was generated by either Marangoni or natural convection. It is often noted that this mechanism leads to an increase in the number of crystals that form in the equiaxed zone. Thus, if one crystal nucleates and begins to grow, it spontaneously fragments under the action of fluid flow, and the fragments then also start to grow. The fragmentation thus leads to one nucleation event spawning numerous growing crystals. This effect is also seen when one has strong fluid flow across a dendritic array. For example, during melting crystal fragmentation leads to mass transfer from the solid phase to the hottest temperature in the system and enhanced melting rates. During growth, this phenomena is observed to lead to the formation of an equiaxed zone at temperatures just below the liquidus, where viscosities are low and fluid flow rates are high.
The double hot thermocouple can also be used to study environmental effects on solidification. For example, in Figure 8, the precipitation of a high-temperature phase due to the introduction of water vapor to the sample is shown.
| Table I. Microscope Specifications | |
|---|---|
| Characteristic | Specification |
| Resolution | 0.30 µm (1LM21W), 0.25 µm (1LM21H), field lens:at 100 times (NA=0.95) |
| Scanning Speed | Horizontal:15.73 kHz, Vertical: 60Hz, 2:1 Interlace |
| Light Source | Laser RED:He-Ne 632.8 µm (light source output 1.5 mW at maximum 0.1 mW when assembled) Normal lighting: Halogen lamp |
| Output Signal | (VIDEO SYNC) Composite |
| Frame Memory | 910*485*8 bi*2 pieces (for images and surface profiling) |
| Zoom | Electronic zoom *2, *4 |
| Slow Scanning | 2,4,8,32,64,128,256,512 (reduces scanning speed at magnifications shown left when normal is set at 1.) |
| Critical Dimension | Measuring range: 0.30 µm or above (1LM21W), 0.25 µm or above (1LM21H) |
| Measurement | Repeatability (3 ): 0.03 µm ): 0.03 µm |
| Surface Profiling | Measuring range: 0.1 µm to 5.7 µm (6.5 mm optionally available) Repeatability (3 ): 0.03 µm ): 0.03 µm |
| Instrument Size | Camera head: 105*350*140 (W*H*D) mm 4.7 kg Control unit: 420*105*490 (W*H*D) mm 13.0 kg Monitor: 320*322*356 (W*H*D) mm 9.0 kg YM mirror base: Approx. 56.0 kg (according to specifications) |
| Power Source | AC115V 50/60Hz: A maximum of 8 A power supply is required when mounting full range of options |
| Power Consumption | Camera head: supplied by the control unit Control unit: 150 W at maximum Monitor: Approximately 37 W |
Although confocal microscopy has been used quite extensively in biological research,14 it is only recently that confocal optics combined with a laser has been utilized for metallurgical studies. To the best of the authors' knowledge, there are currently only two systems in the world that are equipped to study phenomenon under steel-making conditions; these are located in Tohoku University (Sendai, Japan) and the University of Wollongong (Australia). Work related to clean-steel production and other metallurgical applications has been pioneered and developed by T. Emi, formerly at Tohoku University (Japan) and currently SIPA professor at the Royal Institute of Technology, and co-workers.
Real-time investigation of crystal growth in the Fe-C alloys were carried out in CSLM by observing the melt surface under argon with a temperature gradient of a few K · mm–1 across the surface.15 The investigators were able to observe and measure, among other things, that the limits in the growth rate when  -solidification resulted in transitions from planar to cellular and then dendritic growth; planar to cellular transition occurs through sequential perturbations at 30–65 µm intervals in the advancing front; and the cell-tip stability observed in Fe-83C was in good agreement with the theory proposed in literature, and that nonmetallic inclusions could be seen to sometimes get engulfed into the solidifying front.
-solidification resulted in transitions from planar to cellular and then dendritic growth; planar to cellular transition occurs through sequential perturbations at 30–65 µm intervals in the advancing front; and the cell-tip stability observed in Fe-83C was in good agreement with the theory proposed in literature, and that nonmetallic inclusions could be seen to sometimes get engulfed into the solidifying front.
Direct observations of collision, agglomeration, and cluster formation of Al2O3 and CaO-Al2O3 inclusions on the surface of molten-steel samples where carried out under argon atmosphere16,17 at 1,673 K. It was concluded in the case of Al2O3-Al2O3 particle interaction that clustering occurred sequentially through the formation of intermediate aggregates, loose structured dendrites, and finally densification to more compact units through sintering. A strong long-range attraction (10–16 N) due to a capillary attraction force between the solid particles and the gas/melt interface was responsible for the agglomeration. The magnitude and reach of the force increased with increasing Al2O3 particle size, but was not affected by the bulk chemistry of the steel melt. In the case of CaO-Al2O3 and CaO-Al2O3-SiO2 inclusions, it was found that for solid particles, the collision was caused by capillary attraction (as in the case of Al2O3- Al2O3 particles); when a liquid inclusion touched a solid cluster, it would spread and cover the solid cluster; and liquid particles did not attract each other due to capillary attraction and merged together only by being pushed by the solidifying front. In all cases thermocapillary flow was found not to play an important role in agglomeration or cluster formation.
The solubility of manganese and sulfur in Fe-Ni melts were studied by observing the formation of MnS on the surface of Fe-Ni alloys with CSLM.18 MnS was found to precipitate heterogeneously on Al2O3 inclusions and grow to either pyramidal- or rod-like shapes. Since the cooling rate did not have a strong influence on the nucleation temperature and since the temperatures for nucleation and dissolution (during heating) were close, the reaction was considered close to equilibrium. The equilibrium constant calculated from the CSLM observations were, however, somewhat larger than what had been reported in literature.
CSLM was also used for studying the interaction of nonmetallic inclusion particles with the advancing melt/solid steel interface.19 Beyond a critical-growth velocity, the solidifying front engulfed the particle by forming a bump perturbing out of the interface. The critical velocity above which engulfment occurred and below which particle pushing occurred for a particle with radius R was found to be v = 60/R for solid particles and 23/R for liquid particles.
Recently, Dippenar et al.20 reported an in-situ study of the solidification of steel. The relation between cell-tip radius and the growth rate required to form dendrites during solidification of carbon steel that was found through CSLM was in agreement with theoretical predictions. Morphological instability and finger-like growth was observed during the solid state  to
to  transformation. Furthermore, the peritectic reaction rate in carbon steel was measured, and the rate-limiting step was attributed to carbon diffusion.
transformation. Furthermore, the peritectic reaction rate in carbon steel was measured, and the rate-limiting step was attributed to carbon diffusion.
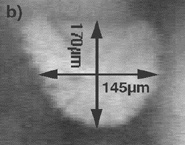
Figure 11's video clip illustrates the solidification of a carbon-saturated iron sample observed from the top. The crystal density varied with undercooling, with the expected outcome that a higher undercooling resulted in higher grain density. The film, however, shows the case for a relatively low undercooling, thus allowing the grain evolution more time before impingement. Nonmetallic inclusions are pushed ahead of the evolving grains.
 |
 |
| a | b |
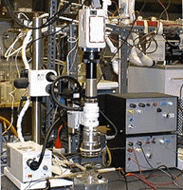
| Figure 15. (a) A schematic and (b) photograph of the experimental setup for interfacial heat transfer behavior. | |
After solidification, the sample contained drops of a liquid phase on the solid-metal surface. The liquid drops could be either a low melting slag phase or highly segregated molten liquid. The fact that they were semitransparent, however, suggests that it may be a slag phase. Figure 13 shows the surface after solidification, when most of the liquid drops have disappeared. A solid-state transformation front proceeds, starting from top left.
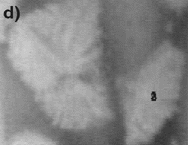
The sample is shown in Figure 14 after solidification, with various nonmetallic inclusions clearly visible on the surface. An almost perfectly spherical particle can be seen on the upper lefthand corner. A plate- or rod-like phase is seen to occur with a certain periodicity, originating from a grain boundary. It is possible that this is a carbide phase. This film shows the growth of one of these phases. Thus, CSLM can be used to track the formation of precipitates in liquids as well as solids in real time and real conditions.
 |
| a |
 |
| b |
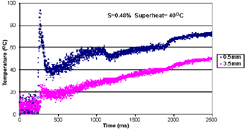
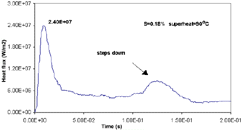
| Figure 16. (a) Measured mold and chill temperatures vs. time and (b) heat flux vs. time at 90° superheat for droplet solidification studies. |
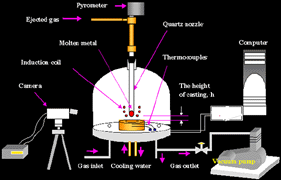
Various investigators have measured rapid heat transfer between solidifying metals and copper molds by monitoring the surface temperature of the casting with a pyrometer21–23 or a photo diode sensor.24 L. Strezov et al.25 investigated the initial heat-transfer behavior using a substrate embedded in a inclined moving paddle similar to melt/roll contacting geometry of the meniscus region of a twin-roll caster. Measurements in a prototype twin-roll caster and laboratory strip casters have also been conducted.26–28 While considerable uncertainties remain in properly understanding and predicting the rapid heat transfer, it has been shown that:
 |
In order to conduct the measurement properly, it is critical to control the fall of the droplet. The process had to be monitored continuously in real time and conditions to ensure that the fall was occurring as expected; a video recording system was designed to record the process as it happened. An example of the process can be seen in the film on Figure 17. It is noteworthy that the shell solidification front and thermal front can be studied with this technique. This novel approach of simultaneous in-situ observation and measurement of rapid heat transfer enables a coupling between the interfacial heat transfer and droplet solidification.
References
1. W.A. Tiller, The Science of Crystallization: Microscopic Interfacial Phenomena (New York: Cambridge University Press, 1991).
2. W.A. Tiller, The Science of Crystallization: Macroscopic Phenomena and Defect Generation (New York: Cambridge University Press, 1991).
3. W. Kurtz and D.J. Fisher, Fundamentals of Solidification (Clausthal-Zellerfeld, Germany: Trans Tech Publications, 1986).
4. F. Ordway, J. Res. Natl. Bur. Stand., 48 (1952), p. 512.
5. J.H. Welch, J. Sci. Instrm., 31 (1954), p. 458.
6. T. Yanagase et al., Proc. of Inter. Conf. Sci. and Tech. Iron and Steel (Tokyo: ISIJ, 1971), p. 516.
7. Y. Ohta, K. Morinaga, and T. Yanagase, Bulletin Japan Inst. Metal., 19 (1980), p. 139.
8. E. Asayama, H. Takebe, and K. Morinaga, ISIJ International, 33, (1993), p. 233.
9. Y. Kashiwaya, D. Jukawa, and K. Ishii, JASMAC-8 (1992).
10. Y. Murayama, Y. Kashiwaya, and K. Ishii, CAMP-ISIJ, 7(1994), p. 891.
11. T. Kuranaga, Y. Kashiwaya, and K. Ishii, Int'l. Symp. on Adv. Mat. and Tech. for 21st Cent. (Sendai, Japan: JIM, 1995).
12. Y. Kashiwaya, C.E. Cicutti, and A.W. Cramb, ISIJ International, 38 (1998) (4), p. 357.
13. Y. Kashiwaya et al., ISIJ International, 38 (4) (1998), pp. 348–356.
14. C.J.R. Sheppard, Confocal Laser Scanning Microscopy (Heidelburg, Germany: Springer-Verlag, 1997).
15. H. Chikama et al., Materials Transactions, JIM, 37 (4) (1996), pp. 620–626.
16. H. Yin et al., ISIJ International, 37 (10) (1997), pp. 936–945.
17. H. Yin et al., ISIJ International, 37 (10) (1997), pp. 946–955.
18. N. Yuki, H. Shibata, and T. Emi, ISIJ International, 38 (4) (1998), pp. 317–323.
19. H. Shibata et al., ISIJ International, 38 (2) (1998), pp. 149–156.
20. R.J. Dippenaar, H. Shibata, and T. Emi, Materials 98, Vol. 2, Engineering Materials (Wollongong, Australia: Wollongong University Press, 1998), pp. 445–450.
21. H. Mizukami, T.Suzuki, and T. Umeda, Tetsu-to-Hagane, 77, No.10 (1991), pp. 1672–1679.
22. W. Liu, G.X. Wang, and E.F. Matthys, Int. J. Heat Mass Transfer, 38 (8) (1995), pp. 1387–1395.
23. G.X. Wang and E.F. Matthys, "On the Heat Transfer at the Interface Between a Solidifying Metal and a Solid Substrate," Melt Spinning, Strip Casting and Slab Casting, ed. E.F. Matthys and W.G. Truckner (Warrendale, PA: TMS, 1996), pp. 205–237.
24. H. Todoroki et al., Proc. of Electric Furnace Conf. (Warrendale, PA: ISS, 1996), pp. 585–590.
25. L. Strezov and J.Herbertson, ISIJ International, 38 (9) (1998), pp. 959–966.
26. K. Miyazawa et al., Int. Conf. On New Smelting Reduction and Near Net Shape Casting Technologies for Steel, vol. 2 (Pohang, Japan: ISIJ, 1990), pp. 745–750.
27. J.C. Grosjean et al., I&SM, (20 August 1993), pp. 27–32.
28. T. Mizoguchi and K.Miyazawa, Proc. of the First European Conf. on Adv. Materials and Processes, vol. 1 (Aachen, Euromat, 1989), p. 93–99.
29. H. Todoroki et al., "Solidification of Steel Droplets Against a Water Cooled Copper Mold," Proc. of the McLean Symposium (Warrendale, PA: ISS, 1998), pp. 155–175.
30. H. Todoroki, Y. Suzuki, and A.W. Cramb, "Initial Solidification of Iron, Nickel and Steel Droplets," I&SM, 26 (4) (1999), pp. 57–71.
31. H. Todoroki et al., "Initial Solidification of Iron, Nickel and Steel Droplets" (Paper presented at the 1998 TMS Annual Meeting, San Antonio, TX, 15150;19 February 1998).
C. Orrling, Y. Fang, N. Phinichka, S. Sridhar, and A.W. Cramb are with the Department of Materials Science and Engineering at Carnegie Mellon University.
For more information, contact A.W. Cramb, Carnegie Mellon University, Department of Materials Science and Engineering, Pittsburgh, Pennsylvania 15217; (412) 268-2964; e-mail cramb@andrew.cmu.edu.
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|