

 |
|
|---|---|
| Previous Session | Next Session |
| Return To Program Contents Page | |
Room: Salon 8
Location: Clarion Plaza Hotel
Session Chairperson: Charles O. Bounds, Rhone-Poulenc Rare Earths and Gallium, Cranbury, NJ 08512; Danesh Chandra, Dept. of Chemical and Metallurgical Engineering, University of Nevada, Reno, NV 89557
SURFACE FILMS ON MmNi.5.5Co0.8Al0.7 ELECTRODES IN 30% KOH THEIR IMPACT ON HYDROGEN STORAGE CAPACITY AND REACTION RATES: M.E. Fiorino, Bell Laboratories, Lucent Technologies, Whippany, NJ 07981; R.L. Opila, Bell Laboratories, Lucent Technologies, Murray Hill, NJ 07974; K. Konstadinidas, Bell Laboratories, Lucent Technologies, Norcross, GA 30071, W.C. Fang, Utmost Industrial Corp., Taipei, Taiwan, China
Misch metal based intermetallics are currently employed as reversible hydride-forming electrodes in Ni - Metal Hydride batteries with 30% KOH electrolyte. In spite of the thermodynamic instability of rare earths in this medium, these electrodes retain storage hydrogen ability and support rapid electron transfer for hundreds of hydriding-dehydriding cycles. Results of electrochemical and XPS studies of one such intermetallic MmNi3.5Co0.8Al0.7, have led to the development of a model to explain these properties.
2:00 pm
THERMAL AGING OF LaNi5-xMnx HYDRIDES: S. Bagchi, D. Chandra, W.N. Cathey, University of Nevada, Reno, NV 89557-0136 USA, R.C. Bowman Jr., Aerojet Electronic Systems, P.O. Box 296, Azusa, CA 91702; F. E. Lynch, Hydrogen Consultant Inc., 12420 N. Dumont Way, Littleton, CO 80125
The Joule-Thomson expansion of hydrogen gas offers a method to produce temperatures below 30K for use in cryocollers in space surveillance satellites. Metal hydrides are a critical components of these devices, providing a non-mechanical method to compress the hydrogen gas. The LaNi5-xMnx-hydrides have potential applications in cryocooling devices: Luo and coworkers1 presented detailed studies on their hydriding behavior. In this study, thermal aging behavior of two LaNi-5-xMnx-hydrides (x=0.4, and 1.5) was investigated to evaluate its long term stability. The hydriding behavior of La1.02Ni4.6Mn0.4 did not change significantly after thermal aging at 453K for 260 hours.
2:30 pm
EFFECT OF RARE EARTH SUBSTITUTIONS FOR La ON THE HYDROGEN ABSORPTION OF La-BASED AB5 ALLOYS: C.O. Bounds, B.M. Ma, J. Patel, Rhône-Poulenc, Rare Earths and Gallium, CN 7500, Cranbury, NJ
The performance of Ab5-type alloys for hydrogen absorption including the electrochemical absorption as an electrode for rechargeable batteries has been demonstrated to be a complex function of the composition, microstructure and surface condition. The compositional studies have focused primarily on substitutions for Ni on the B-site (e.g. Co, Al, Mn, etc.). This current study examines the effect of rare earth substitutions for La on the A-site and considers both other "light" rare earths (Ce, Nd, Pr) and "heavy" rare earths (Dy, Sm, etc.).
3:00 pm BREAK
3:30 pm
GAS-PHASE HYDROGENATION PROPERTIES AND ELECTRODE PERFORMANCE OF La(Ni,Al,Co,V)5 ALLOYS: L.-C. Lei, S.-U. Liu T.-P. Perng, Department of Materials Science and Engineering, National Tsing Hua University, Hsinchu, Taiwan, 30043
A series of LaNi5-based alloys, with partial substitution of Ni by Al, Co, or V, were prepared. The gas-phase hydrogen absorption kinetics and pressure-Composition-temperature curves were measured. The activation became easier and the plateau pressure was reduced as Ni was partially substituted by Al. Substitution with Co reduced the hysteresis as well as the hydrogenation capacity. Less degradation of hydrogenation capacity after long cycles of hydriding- dehydriding was observed when substituted with both Al and Co. Vanadium is often considered as a hydride former, but when it was added to LaNi5 it was found that V occupied the B site. A small amount of substitution of Ni with V led to a higher hydrogenation capacity and faster activation rate.
4:00 pm
ATOMIZATION PROCESSING EFFECTS ON HYDRIDING BEHAVIOR OF AB5 ALLOYS FOR BATTERY APPLICATIONS: R.C. Bowman, Jr., C. Witham, B. Fultz, California Institute of Technology, Pasadena, CA 91125; B.V. Ratnakumar, Jet Propulsion Laboratory, Pasadena, CA 91109; T.W. Ellis, Kulicke and Soffa Industries, Inc., Willow Grove, PA 19090; I.E. Anderson, Ames Laboratory, Ames, IA 51122
Hydriding characteristics of some AB5 alloys produced by high pressure gas atomization (HPGA) were examined during reactions with hydrogen gas, and In electrochemical cells. Hydrogen storage capacities and equilibrium pressures for HPGA LaNi5, LaNi4.75Sn0.25, and MmNi3.5Co0.8Al0.4Mn0.3 were nearly identical to cast alloys after extensive annealing. The large discontinuous volume change across the alpha-beta plateau region for HPGA LaNi5Hx produced extensive fracturing in all but the smallest alloy spheres.
4:30 pm
NEW ANISOTROPIC RARE EARTH LASER FLUORIDES BaR2F8(R=Y,Dy-LU): GROWTH AND CHARACTERIZATION: Alexander A. Kaminskii, Andrei V. Butashin, Institute of Crystallography, Russian Academy of Sciences, Leninskii prosp. 59, 117333, Moscow, Russia
Anisotropic fluoride crystals doped with Ln3+ ions have attracted a great interest of investigators as effective active materials for designing multi-wave crystalline lasers, which emit stimulated emission (SE) in the unique number of the 4fN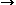 4fN and 4fN-15d1
4fN and 4fN-15d1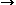 4fN manifold-to-manifold transitions in UV, visible, and IR spectral ranges. Among them BaR2F8 crystals are distinguished by their Congruent melting at
4fN manifold-to-manifold transitions in UV, visible, and IR spectral ranges. Among them BaR2F8 crystals are distinguished by their Congruent melting at  950°C, good capacity to dissolve activator Ln3+ ions, wide transparency ranging from VUV to far IR, birefringence and short phonon spectra.
950°C, good capacity to dissolve activator Ln3+ ions, wide transparency ranging from VUV to far IR, birefringence and short phonon spectra.
| Previous Session Next Session | ||||
|---|---|---|---|---|
| Search | Technical Program Contents | 1997 Annual Meeting Page | TMS Meetings Page | TMS OnLine |