
1997 TMS Annual Meeting: Tuesday Abstracts
STRUCTURE AND PROPERTIES OF INTERNAL INTERFACES: Session III: Interfacial Diffusion and Transformations
Sponsored by: Jt. EMPMD/SMD Chemistry & Physics of Materials Committee, MSD Computer Simulation Committee
Program Organizer: Diana Farkas, Dept. of Materials Science and Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061; Elizabeth A. Holm, Sandia National Lab, Physical and Joining Metallurgy, MS 1411, Albuquerque, NM 87185-0340; David J. Srolovitz, Dept. of Materials Science & Engineering, University of Michigan, Ann Arbor, MI 48109-2136
Room: 330G
Session Chairperson: David N. Seidman, Northwestern University, Materials Science and Engineering Dept./MLSB, 2225 North Campus Drive, Evanston, IL 60208-3108
8:30 am INVITED
TEMPERATURE AND CONCENTRATION DEPENDENCE OF GRAIN BOUNDARY-DIFFUSION OF 63Ni IN PURE AND BORON-DOPED Ni3Al: Stefan Frank, Jörg Rüsing and Christian Herzig, Institut für Metallforschung, Universität Münster, Germany, now: Hahn-Meitner-Institut, Berlin, Germany
Knowledge about the diffusion behaviour in the grain boundaries of pure and of boron-doped Ni3Al is of basic importance to improve our understanding of the boron-effect and the mechanical properties of polycrystalline Ni3Al. We report on a first study of grain boundary diffusion in pure and doped Ni-rich Ni3Al polycrystals. Diffusion penetration profiles were determined by a serial sectioning technique using a precision parallel grinding device. The low energy  -decays of the 63Ni tracer were detected by liquid scintillation counting. The high efficiency allowed to significantly increase the detectable concentration range of the measured profiles and provided the basis for investigating grain boundary self-diffusion in Ni3Al in a meaningful way. For comparison grain boundary self-diffusion in pure Ni was re-investigated in true type R-kinetic. It is known that the temperature dependence of the grain boundary diffusion parameter P=
-decays of the 63Ni tracer were detected by liquid scintillation counting. The high efficiency allowed to significantly increase the detectable concentration range of the measured profiles and provided the basis for investigating grain boundary self-diffusion in Ni3Al in a meaningful way. For comparison grain boundary self-diffusion in pure Ni was re-investigated in true type R-kinetic. It is known that the temperature dependence of the grain boundary diffusion parameter P= Dgb of 63Ni in pure Ni and in both Ni3Al materials is of the Arrhenius type. The absolute values of P follow the sequence P Ni>P Ni3Al>P Ni3Al+B. The remarkably higher activation enthalpy Qgb (Ni3Al) in comparison with Qgh (Ni) can be attributed to the grain boundary structure of the strongly ordered Ni3Al compound. Ni-diffusivity in the doped alloy is about 2-3 times lower than in pure Ni3Al. A prospective reason for the decreased diffusivity is the segregation of B and the thereby enhanced cosegregation of Ni in the grain boundaries. The strong segregation of boron leads to a certain blocking of diffusion paths of the Ni atoms. High angle grain boundary energies in pure and R-doped Ni3Al are derived from the diffusion data applying the Borisov et al.relation. The effect of stoichiometry on grain boundary self-diffusion in Ni3Al was investigated for compositions between 73 and 78 at% Ni. A V-shaped concentration dependence of P with a minimum at near 75 at% Ni was observed which becomes very distinct at lower temperatures. At e.g. 1066 K P increases by about one order of magnitude from stoichiometric to Ni-rich Ni3Al. This behaviour is related to the change in composition and state of order of grain boundaries in non stoichiometric Ni3Al.
Dgb of 63Ni in pure Ni and in both Ni3Al materials is of the Arrhenius type. The absolute values of P follow the sequence P Ni>P Ni3Al>P Ni3Al+B. The remarkably higher activation enthalpy Qgb (Ni3Al) in comparison with Qgh (Ni) can be attributed to the grain boundary structure of the strongly ordered Ni3Al compound. Ni-diffusivity in the doped alloy is about 2-3 times lower than in pure Ni3Al. A prospective reason for the decreased diffusivity is the segregation of B and the thereby enhanced cosegregation of Ni in the grain boundaries. The strong segregation of boron leads to a certain blocking of diffusion paths of the Ni atoms. High angle grain boundary energies in pure and R-doped Ni3Al are derived from the diffusion data applying the Borisov et al.relation. The effect of stoichiometry on grain boundary self-diffusion in Ni3Al was investigated for compositions between 73 and 78 at% Ni. A V-shaped concentration dependence of P with a minimum at near 75 at% Ni was observed which becomes very distinct at lower temperatures. At e.g. 1066 K P increases by about one order of magnitude from stoichiometric to Ni-rich Ni3Al. This behaviour is related to the change in composition and state of order of grain boundaries in non stoichiometric Ni3Al.
9:10 am
CALCULATIONS OF DIFFUSION AND SEGREGATION IN Al-Cu GRAIN-BOUNDARIES: X.Y. Liu, J.B. Adams, Department of Materials Science and Engineering, University of Illinois at Urbana-Champaign, 105 South Goodwin Avenue, Urbana, IL 61801; Wei Xu, Lawrence Livermore National Laboratory, University of California, Livermore, CA 94551
We have studied the atomistic mechanisms of electromigration in Al-Cu, in specific, the diffusion of atoms and segregation of Cu atoms in the Al grain-boundaries with a newly developed EAM interatomic potential constructed by the Force-Matching Method. The potential parameters are fitted to available experimental data plus ab initio force database. The energetic and structural properties of Cu segregation and the effect in atomic diffusion path and activation barriers are studied. The grain-boundary calculations, in agreement with ab initio results, show that the Cu segregation is dominated by size effects in the Al-Cu system, i.e., Cu atoms segregate to sites which are locally compressed. In the case of a 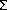 11 tilt boundary, this predicts that the Cu will segregate to sites away from the symmetric center of the boundary.
11 tilt boundary, this predicts that the Cu will segregate to sites away from the symmetric center of the boundary.
9:30 am
ATOMISTIC SIMULATION OF GRAIN BOUNDARY STRUCTURE AND DIFFUSION IN B2 NiAl: Yuri Mishin, Diana Farkas, Virginia Polytechnic Institute and State University, Materials Science and Engineering Department, Blacksburg, VA 24061
We modify the existing embedded-atom potentials for NiAl by fitting them to self-diffusion data for pure Ni and Al and to the triple-defect model for this compound. Using the modified potentials and molecular statics we calculate the structure, excess energy and cohesive energy of [001] tilt grain boundaries in NiAl. The calculations are performed for 25 orientations with $\Sigma$ values from 5 to 185. For each orientation the lowest-energy stoichiometric boundary structure is established. Low-$\Sigma$ orientations are associated with minima of the boundary energy and maxima of the cohesive energy. The boundary structures obtained are analyzed in terms of the structural unit model. For two lowest-energy boundaries, $\Sigma=5$ (210) and $\Sigma=13$ (230), we simulate tracer self-diffusion in the boundary core both parallel and normal to the tilt axis. We assume the vacancy mechanism and calculate the vacancy formation and migration energies in the boundary core. To calculate the boundary diffusion coefficients we use the technique proposed recently by one of us [Phil. Mag. A 72, 1589 (1995)]. This technique combines the matrix method with Monte Carlo simulations of individual vacancy-tracer encounters. The effective activation energy is calculated in a wide temperature range and compared with the spectrum of individual jump energies in the boundary. The results are discussed in terms of the grain boundary mass transport and its effect on high temperature properties of NiA.
9:50 am
THE IVANTSOV GROWTH EQUATION AND INTERFACIAL KINETICS: Zi-Kui Liu, Y. Austin Chang, Department of Materials Science and Engineering, 1509 University Avenue, University of Wisconsin-Madison, Madison, WI 53706
The growth equation of parabloid was first derived by Ivantsov1 and later modified by Trivedi2 to take into account the interfacial energy and finite interfacial mobility (interfacial kinetics). The modification was done by first considering the effects of the interfacial kinetics and the interfacial energy and curvature, and then solving the diffusion equations on the composition at the interface. Though the modification was necessary, the growth equation was much more complicated that the original Ivantsov equation. In a recent publication by us, the advantage of the modern development of computational thermodynamics was taken and the Ivantsov equation was then used directly with the actual composition after taking into account the interfacial energy and the finite interfacial mobility. It was pointed out by a reviewer that this approach was not self evident if it is identical to Trivedi's modification. In the present report, we will formulate a consistent framework to describe the phenomena.
10:10 am
MICROSTRUCTURAL CHARACTERIZATION OF TiAl3 PRODUCT LAYERS IN AlCuSi/Ti AND AlCu/Ti REACTION COUPLES: C. Wauchope, J.E. Sanchez, Jr., University of Michigan, Ann Arbor, MI 48109; P.R. Besser, R. Alvis, Advanced Micro Devices, Sunnyvale, CA 94088
Metallization interconnects in advanced integrated circuits are typically fabricated from Al alloy (500-1000nm)/Ti (20-50nm) multilayer thin films. The Ti provides several effects in the Al layer; improved reliability against electromigration-induced failure, reduced Al grain size, increased Al (111) fiber texture, and reduced Al thickness due to Ti+Al reaction to form TiAl3. The TiAl3 reaction occurs during thermal processing and device fabrication, and leads to increased line resistance as the Al thickness is reduced. We present detailed cross-section transmission electron microscopic analysis of TiAl3 layers formed in AlCuSi/Ti and AlCu/Ti reaction couples. Results show that the Al alloy/TiAl3 and TiAl3/Ti interfaces are "clean" and free of intermediate phase layers. In addition local energy dispersive x-ray spectroscopy (EDS) analyses show Si partitioning in the TiAl3 region in AlCuSi/Ti samples. The TiAl3 grain size is roughly independent of Al alloy type, and depends primarily on total TiAl3 thickness. These results are described in terms of models which describe the effects of Cu and Si alloying additions on the TiAl3 formation kinetics, in particular to the Ti+Al reaction rate dependence on the Ti/AlCuSi thickness ratio.
10:30 am BREAK
10:50 am INVITED
STRUCTURE, COMPOSITION AND THERMAL STABILITY OF METAL/CERAMIC INTERFACES: Manfred Rühle, Max-Planck-Institut für Metallforschung, Seestr. 92, D-70174 Stuttgart, Germany
Metal/ceramic interfaces play a crucial role in different areas of materials science such as metallic interconnects in semiconductors, adhesion of oxide scales on metal substrates, thermal barrier coating, composites and bonding between bulk parts of metals and ceramics. It is of great interest to correlate the microstructure of those interfaces to their properties. The possibilities of getting information on the structure and composition of metal/ceramic interfaces will be discussed for several systems. The focus will be mainly on model systems (Cu/Al2O3, Ni/Al2O3, Nb/Al2O3) but also applications for real materials will be discussed. Segregation of impurities may lead either to an increase or decrease of the adhesion. Therefore, it is crucial to determine those impurities as accurately as possible. Metal/ceramic interfaces are often exposed to high temperatures at different atmospheres. Therefore, the thermal stability under service conditions is of great interest. The paper will describe recent advances and possibilities of determining the structure, composition and chemical reactions at those interfaces by conventional transmission electron microscopy, high-resolution transmission electron microscopy and analytical electron microscopy. The results of experimental studies will be compared to results of ab-initio first principles calculations and to experimental studies on the adhesion of metal films on ceramic substrates.
11:30 am
THE Al/Ni AND Al/Cu INTERFACIAL REACTIONS UNDER THE INFLUENCE OF ELECTRIC CURRENT: Wen-Chyuarn Liu, Sinn-wen Chen, Department of Chemical Engineering, National Tsing-Hua University, Hsin-Chu, Taiwan 30043, China
Electromigration has been observed and quite extensively investigated in the integrated circuit devices with the electric current density higher than 105 A/cm2. The electric current density in the electronic packaging was usually lower and the effect of electromigration has not been discussed. This study investigated the effect of weaker electric current, up to 103 A/cm2, upon the chemically-driven interfacial reactions in the Al/Ni and Al/Cu systems by analyzing their reaction couples. The reaction couples would be heated by the passing-through electric current. The temperatures of the reaction couples increased with the increasing electric density. Annealed at the same temperatures, similar results were found for the reaction couples with and without the passing-through electric current. Same intermetallics, such as Al3Ni and Al3Ni2, were formed at the interfaces, and the thickness of the reaction layers was the same. It was concluded that the electric current density up to 103 A/cm2 has little effect upon the interfacial reactions in the Al/Ni and Al/Cu systems.
 -decays of the 63Ni tracer were detected by liquid scintillation counting. The high efficiency allowed to significantly increase the detectable concentration range of the measured profiles and provided the basis for investigating grain boundary self-diffusion in Ni3Al in a meaningful way. For comparison grain boundary self-diffusion in pure Ni was re-investigated in true type R-kinetic. It is known that the temperature dependence of the grain boundary diffusion parameter P=
-decays of the 63Ni tracer were detected by liquid scintillation counting. The high efficiency allowed to significantly increase the detectable concentration range of the measured profiles and provided the basis for investigating grain boundary self-diffusion in Ni3Al in a meaningful way. For comparison grain boundary self-diffusion in pure Ni was re-investigated in true type R-kinetic. It is known that the temperature dependence of the grain boundary diffusion parameter P= Dgb of 63Ni in pure Ni and in both Ni3Al materials is of the Arrhenius type. The absolute values of P follow the sequence P Ni>P Ni3Al>P Ni3Al+B. The remarkably higher activation enthalpy Qgb (Ni3Al) in comparison with Qgh (Ni) can be attributed to the grain boundary structure of the strongly ordered Ni3Al compound. Ni-diffusivity in the doped alloy is about 2-3 times lower than in pure Ni3Al. A prospective reason for the decreased diffusivity is the segregation of B and the thereby enhanced cosegregation of Ni in the grain boundaries. The strong segregation of boron leads to a certain blocking of diffusion paths of the Ni atoms. High angle grain boundary energies in pure and R-doped Ni3Al are derived from the diffusion data applying the Borisov et al.relation. The effect of stoichiometry on grain boundary self-diffusion in Ni3Al was investigated for compositions between 73 and 78 at% Ni. A V-shaped concentration dependence of P with a minimum at near 75 at% Ni was observed which becomes very distinct at lower temperatures. At e.g. 1066 K P increases by about one order of magnitude from stoichiometric to Ni-rich Ni3Al. This behaviour is related to the change in composition and state of order of grain boundaries in non stoichiometric Ni3Al.
Dgb of 63Ni in pure Ni and in both Ni3Al materials is of the Arrhenius type. The absolute values of P follow the sequence P Ni>P Ni3Al>P Ni3Al+B. The remarkably higher activation enthalpy Qgb (Ni3Al) in comparison with Qgh (Ni) can be attributed to the grain boundary structure of the strongly ordered Ni3Al compound. Ni-diffusivity in the doped alloy is about 2-3 times lower than in pure Ni3Al. A prospective reason for the decreased diffusivity is the segregation of B and the thereby enhanced cosegregation of Ni in the grain boundaries. The strong segregation of boron leads to a certain blocking of diffusion paths of the Ni atoms. High angle grain boundary energies in pure and R-doped Ni3Al are derived from the diffusion data applying the Borisov et al.relation. The effect of stoichiometry on grain boundary self-diffusion in Ni3Al was investigated for compositions between 73 and 78 at% Ni. A V-shaped concentration dependence of P with a minimum at near 75 at% Ni was observed which becomes very distinct at lower temperatures. At e.g. 1066 K P increases by about one order of magnitude from stoichiometric to Ni-rich Ni3Al. This behaviour is related to the change in composition and state of order of grain boundaries in non stoichiometric Ni3Al.

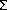 11 tilt boundary, this predicts that the Cu will segregate to sites away from the symmetric center of the boundary.
11 tilt boundary, this predicts that the Cu will segregate to sites away from the symmetric center of the boundary.