

 | |
|---|---|
| Previous Session | Next Session |
| Return To Program Contents Page | |
Room: 231B
Session Chairpersons: R.G. Bautista, Mackay School of Mines, University of Nevada, Reno, NV 89557: G. Ramadorai, EnMet Associates Inc., 11225 E. Quick Draw Place, Tucson, AZ 85749
2:30 pm
PRODUCTION OF THERMAL SPRAY-QUALITY METAL AND ALLOY POWDERS BY PLASMA PROCESSING: P.V. Ananthapadmanabhan, K.P. Sreekumar, N. Venkatramani, Laser & Plasma Technology Division, Bhabha Atomic Research Centre, Bombay-400 085, India; Patrick R. Taylor, Department of Mining & Metallurgical Engineering, University of Idaho, Moscow, ID 83844
Feedstock powders of metals, alloys and ceramics for thermal spray applications have to meet several specifications. Particle shape, size and distribution, powder flow characteristics and density are the important factors that need to be controlled, in order to ensure high spray efficiency and better coating properties. Thermal plasma technology can be effectively utilized to produce metal, alloy and ceramic powders for spray applications. The present paper describes plasma spheroidisation of commercially available aluminum powder and nickel-aluminum powder blend in a plasma reactor. Results show that the processed powder particles bear spherical morphology with excellent flow characteristics, ideal for thermal spray applications.
2:55 pm
NON-ELECTROLYTIC DEPOSITION OF SILVER ON TO TUNGSTEN-POWDER PARTICLES: Jae-Ho Lee, Hong lk University, Department of Metallurgy and Materials Engineering, 721 Sangsu dong, Mapo-gu, Seoul 121-791, Korea; G.P. Martins, Colorado School of Mines, Department of Metallurgical and Materials Engineering, Golden, CO 80401
For some electronic applications where a dispersed electrically-conductive particulate phase is employed, the conductivity of the surface (or near-surface region) of the particles provides for the primary mechanism which determines its electrical conductivity. Particles of a less expensive material, which may have desirable thermal-expansion properties, when coated with silver offer a means of also obtaining desirable electrical properties at a lower cost. The research conducted was focused primarily on the development of silver-coated tungsten particles for thick-film polymeric conductors. The ammoniacal electrolyte was formulated from silver-nitrate, glycine inhibitor and formaldehyde reductant. The reduction, and subsequent deposition, of silver occurred selectively on the surface of the tungsten particles. Coated particles were assessed by SEM imaging. The thickness of the silver coating was estimated to be approximately 100nm on the basis of a mass account and the coating being uniform. The electrical conductivity of a silver-coated tungsten-powder pellet was found to be similar to that of a silver-powder pellet, of identical geometry.
3:20 pm
VISCOSITY MODELING OF TERNARY ALUMINOSILICATE MELTS: Z. Zhang, R.G. Reddy, The Department of Metallurgical and Materials Engineering, The University of Alabama, Tuscaloosa, AL 35487-0202
Viscosity of CaO-aluminosilicate ternary melts were estimated using a structure based model. The model considers depolymerization effects and related breakdown of the silicate network structure on the addition of metal oxides to the melts. The predicted values are in good agreement with the experimental values over the whole temperature and composition range.
3:45 pm
COPPER DEOXIDIZATION BY BUBBLING HYDROGEN/NITROGEN MIXTURES THROUGH THE MELT: Claudio Parra De Lazzari, Jose Deodoro Trani Capocchi, Department of Metallurgical and Materials Engineering, Escola Politecnica de Universidade de Sao Paulo, Sao Paulo SP-Brazil
The determination of the governing step of the deoxidization rate and the effects of the hydrogen content of the mixtures (C), the diameter of the delivery orifice (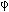 ) and the Reynolds number of the orifice (Roe) on the product Av keg was investigated. Av is the total surface area of the interface between the bubbles and the melt keg, is the mass transfer coefficient in the gas phase. The design of the experiments was based on a two levels statistic fractionated factorial planning Oxygen in the melt was measured as a function of time. In the prevailing experimental conditions it was found that the governing step of the deoxidization rate was the transport of hydrogen in the gaseous phase and that Av kejg increases with C,
) and the Reynolds number of the orifice (Roe) on the product Av keg was investigated. Av is the total surface area of the interface between the bubbles and the melt keg, is the mass transfer coefficient in the gas phase. The design of the experiments was based on a two levels statistic fractionated factorial planning Oxygen in the melt was measured as a function of time. In the prevailing experimental conditions it was found that the governing step of the deoxidization rate was the transport of hydrogen in the gaseous phase and that Av kejg increases with C, 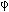 and Reo.
and Reo.
4:10 pm BREAK
4:20 pm
CHEMICAL WEAR OF REFRACTORIES IN THE SLAG LINE OF VOD LADLES FOR THE PRODUCTION OF STAINLESS STEELS: B. Blanpain, P. Wollants, Department of Metallurgy and Materials Engineering, K.U. Leuven, de Croylaan 2, B-3001 Leuven, Belgium, and B. Hallemans, ALZ, Genk-Zuid zone 6A, B-3600 Genk, Belgium
The slag line in vacuum oxygen decarburization (VOD) ladles is a severe environment for refractory materials during the refining of stainless, steels. Magnesia-chrome bricks are currently the refractory materials of choice for this application. The actual performance of a fixed quality of magnesia-chrome bricks is however largely dependent on the conditions during the VOD process cycle. This presentation addresses the aspects of chemical interaction between the slag phase and the magnesia-chrome refractory materials. As-delivered and postmortem bricks have been analyzed by optical microscopy, x-ray diffraction and scanning electron microscopy. Different phenomena were observed such as slag penetration and oxide reduction. These experimental observations are the basis for a modeling of the slag/magnesia-chrome reaction. The aim is to investigate alternate, viable VOD processing routes which are less demanding on the refractory materials.
4:45 pm
PHASE EQUILIBRIA IN THE OXIDE-SATURATED Ca-Mg-O SYSTEM: Xiaoping Xu, Mark E. Schlesinger, Department of Metallurgical Engineering, University of Missouri-Rolla, 1870 Miner Circle, Rolla, MO 65409-0340
Recent research results have claimed that previously accepted Gibbs energies of formation of CaO and MgO are in error, and that MgO is actually more stable than CaO at lower temperatures. The potential impact of this on the existing thermodynamic database and on calculations using  G0f values for these oxides is considerable. However, the newly calculated values remain controversial. Equilibration of pure CaO and MgO with a metal phase at temperatures between 1100°C and 1200°C allows the new
G0f values for these oxides is considerable. However, the newly calculated values remain controversial. Equilibration of pure CaO and MgO with a metal phase at temperatures between 1100°C and 1200°C allows the new  G0f values to be "tested," using a previously derived thermodynamic model for liquid CaMg alloys to calculate activities in the metal phase. The fit between actual and predicted alloy compositions determines the likelihood that the new thermodynamic values are valid.
G0f values to be "tested," using a previously derived thermodynamic model for liquid CaMg alloys to calculate activities in the metal phase. The fit between actual and predicted alloy compositions determines the likelihood that the new thermodynamic values are valid.
5:10 pm
REDUCTION OF MnO PELLETS BY CARBON-SATURATED LIQUID IRON: Jose Roberto de Oliveira, Marcelo Breda Mourdo, Paulo dos Santos Assis and Jorge Alberto Soares Tenorio, Dept. of Metallurgical and Materials Engineering, University of Sao Paulo, 05508-900, Sao Paulo, Brazil
The goal of this study was to investigate the reduction of MnO pellets by carbon saturated liquid iron. The effects of the initial Mn and Si content in the bath were explored. The tests were performed in laboratory apparatus, where MnO pellets reacted with a saturated liquid iron bath. The reduction time was taken by measurement of the CO pressure variation with the time. The results showed the effect of initial Mn, C and Si content in the bath. The Kinetics of MnO reduction increases with the decrease in the initial MnO content in the bath. Si in the bath increases the reduction rate of the MnO pellets.
| Previous Session Next Session | ||||
|---|---|---|---|---|
| Search | Technical Program Contents | 1997 Annual Meeting Page | TMS Meetings Page | TMS OnLine |