

 |
|---|
| Return To Program Contents Page |
Room: 240B
Session Chairperson: George Demopoulos, Dept. of Mining and Metallurgical Engineering, McGill University, Montreal, Quebec, Canada H3A 2A7
2:00 pm
A PYRIDINE-BASED TRIDENTATE CHELATING SOLVENT EXTRACTION SYSTEM FOR SELECTIVE EXTRACTION OF NICKEL AND COBALT: Batric Pesic1, and Taili Zhou2, 1University of Idaho, College of Mines-McClure Hall, Moscow, ID 83843; 2The Shepherd Chemical Co., 4900 Beech St., Cincinnati, OH 45212
A novel pyridine-based tridentate chelating extractant, 2,6bis-[5-n-nonylpyrazo-3-yl] pyridine (BNPP), has been developed and characterized. The solvent extraction of Ni and Co by a mixed system of BNPP and dinonyl naphthalene sulfonic acid (DNNSA) was studied as a function of pH, diluent, temperature, and DNNSA concentration. Stripping of Ni and Co was examined as a function of HCl and H2SO4 concentration. The novel system can extract Ni and/or Co selectively against Fe, Mn, Ca, Mg, and Al from acidic sulfate solutions at a pH as low as 0.5. Separation of Ni and Co can be achieved either during loading, or during stripping stages of solvent extraction. The extractant system is stable and can be regenerated with acid. The novel solvent extraction system was also tested on the real solutions produced by leaching of cobalt bearing concentrates, (1) cobaltite concentrate from Blackbird Mine, Idaho, and (2) siegenite concentrate from Lead-Belt, Missouri. A flowsheet for recovery of cobalt and nickel has been proposed based on these studies. The review of the current status of solvent extractants for cobalt and nickel will be given by comparative presentation with our novel extractantion system.
2:20 pm
DIELECTRIC STUDIES ON PbS-KEX-K2Cr207 SYSTEM UNDER FLOTATION CONDITIONS: Antonio Huerta, Juan Genesca, Armando Solis, Dept. de Ingenieria Metalurgica, Fac.de Qulmica, Universidad Nacional Autonoma de Mexico. Cd. Universitaria, CP.04510, Mexico
The effects of potassium ethyl xanthate used as collector and dichromate potassium a depressor on a PbS mineral were studied by the recently accepted technique in Mexico, high frequency impedance (transformed to dielectric values). Results of dielectric values at 100 MHz suggested that is possible to obtain from isotherm adsorption the adequate industrial collector concentration being this in the range of 1E-3 to 5E-3M of KEX. The e' values (320-420) in the PbS-KEX system was increased as the KEX concentration was lowered). In the other hand the PbSK2Cr207 system showed higher values of e' (420-510) as the K2Cr207 concentration was increased. The PbS-KEX-K2Cr207 system was evaluated, obtaining the KEX concentration dominion on K2Cr207.
2:40 pm
GYPSUM CRYSTALLIZATION IN ACIDIC WASTEWATER TREATMENT: A REVIEW OF CURRENT PRACTICE AND A VIEW ON PROCESS IMPROVEMENT: S. Omelon, G.P. Demopoulos, McGill Department of Mining and Metallurgical Engineering, 3450 University St., Montreal, Canada H3A 2A7
Acidic sulphate-containing wastewater streams are currently neutralized with lime before discharge to the environment. The most common neutralization process design involves a one-step neutralization followed by a settling tank that separates an ultra-fine, low solids density gypsum by-product. The final gypsum quality is undesirable as it is voluminous and has a high surface area that sorbs metal ions. The paper will critically review the current practice of gypsum precipitation. It will also report the progress of a project which uses crystallization principles and supersaturation control to develop a new process. The new process design utilizes a model that was constructed to predict gypsum solubilities in H2SO4-ZnSO4-FeSO4-MnSO4-MgSO4 solutions from 25-60°C. By maintaining a low gypsum supersaturation with a series of CSTR's and providing gypsum seed with a solids recycle, the generation of high solids density gypsum is favoured. Early results will be presented, as well as results on Zn2+ adsorption.
3:00 pm
ANALYSIS OF THE HEMATITE PRECIPITATION PROCESS FROM A CRYSTALLIZATION POINT OF VIEW: T.C.-M. Cheng, G.P. Demopoulos, McGill Department of Mining and Metallurgical Engineering, 3450 University St., Montreal, Canada H3A 2A7
Iron removal and disposal in zinc and nonferrous hydrometallurgical plants at large constitutes one of the major environmental challenges to the industry. So far three technologies have been used by the zinc industry to effect iron rejection, namely the Jarosite, the Goethite, and the Hematite Processes. Among them, it is the Jarosite Process that has dominated the scene. However jarosite residues tend to be voluminous (only 25% Fe content) and highly toxic due to incorporation of Zn, Cd, Pb, and other heavy metals, not to mention the relatively high Zn losses. The industry addresses the heavy metal release problem by further stabilizing jarosite prior to disposal as is done, for example, in the Jarofix Process. On the other hand, hematite residues produced by the Hematite Process have a very high iron content and low zinc losses but come with a relatively high operating cost. At present, commercial produced hematite residues are still considered a waste because of the impurities contained in them whereas the two major troublesome impurities are sulfur and zinc. Hematite technology may become more attractive if the produced hematite is clean enough to be used as a feed material for iron-steel making. In this paper, the practice of industrial hematite precipitation and the relevant technical literature are reviewed with the objective to identify the critical processing parameters which are responsible for the contamination of hematite with sulfur and zinc. The analysis is done from a crystallization theory standpoint. The role of supersaturation control or lack of it is emphasized in the analysis. Finally, the main elements of a research program focus ing on crystallization of hematite that is currently underway at McGill University will be outlined.
3:20 pm BREAK
3:30 pm
INFLUENCE OF HYDROLYSIS--PRECIPITATION MEDIUM ON THE NATURE OF ALUMINIUM HYDROXIDE GELS AND Al203 POWDER CHARACTERISTICS: M. Thiruchitrambalam, V.R. Palkar*, V. Gopinathan, P. Ramakrishnan, M.S. Multani*. Dept. of Metallurgical Engineering and Materials Science, I.I.T, Bombay - 76; *Tata Institute of Fundamental Research, Bombay -5, India
Aluminium hydroxide gels have been widely used in applications like sorbents and catalyst supports. Thermal treatment of aluminium hydroxide gels i.e., calcination and/or sintering, first leads to dehydration and then a series of phase changes. Once dehydration is complete several transition aluminium oxides namely 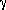 ,
,  and
and  appear, and finally
appear, and finally  Al2O3 is formed at about 1200°C. In the current investigation boehmite (AIOOH) a bayerite (Al(OH)3) have been prepared in the presence of water-glycerol solutions. The results indicate that the precipitation media have considerable influence on the nature of aluminium hydroxide precipitate. Aluminium hydroxide gels thus prepared were characterised by TEM and XRD. Calcined powders were examined by XRD for phase content, by SEM for morphology and BET method for specific surface area. Calcination of aluminium hydroxide gels prepared by Hot water hydrolysis-Controlled precipitation technique yielded agglomerate free, spherical a Al2O3 powder. Al2O3 was also prepared by calcining the aluminium hydroxide gels at 600°C and characterised by XRD, TEM and BET method.
Al2O3 is formed at about 1200°C. In the current investigation boehmite (AIOOH) a bayerite (Al(OH)3) have been prepared in the presence of water-glycerol solutions. The results indicate that the precipitation media have considerable influence on the nature of aluminium hydroxide precipitate. Aluminium hydroxide gels thus prepared were characterised by TEM and XRD. Calcined powders were examined by XRD for phase content, by SEM for morphology and BET method for specific surface area. Calcination of aluminium hydroxide gels prepared by Hot water hydrolysis-Controlled precipitation technique yielded agglomerate free, spherical a Al2O3 powder. Al2O3 was also prepared by calcining the aluminium hydroxide gels at 600°C and characterised by XRD, TEM and BET method.
3:50 pm
CONTINUOUS BACTERIAL LEACHING OF A LOW-GRADE MANGANESE DIOXIDE ORE: S. Agatzini Leonardou, J.G. Zafiratos, Laboratory of Metallurgy, Department of Mining and Metallurgical Engineering, National Technical University of Athens, Athens, Greece
The leaching of a Greek low-grade manganese dioxide ore, not amenable to conventional mineral processing operations, was studied using a mixed culture of Thiobacillus sp. bacteria. The experiments were carried out in specially designed continuously aerated and stirred tank reactors arranged in two-tank series. The first tank of each series was loaded with an elemental sulfur culture suspension while the second was loaded with a manganese ore high density slurry. The bacterial culture had been previously adapted to grow on elemental sulphur and also in the presence of manganese ore and dissolved manganous ion. A two level full factorial design, was constructed in order to study the effects of the ferric ion concentration in the leach solution, the "elemental sulphur weight to ore weight" ratio, the ore pulp density and the composition of the dispersed gas. The responses which were investigated included percentage manganese recovery, co-dissolution of iron and manganese dissolution rate. Parameters with constant values throughout the experiment were the leach solution pH, the nutrient medium composition, the dilution rate and the ore and sulphur grain sizes. It was found that the ferric ion concentration was the most significant factor and had a positive effect on the final percentage extraction of manganese from the ore and also on the rate of manganese solubilisation from the ore. The effects of the other factors studied are discussed in detail.
4:10 pm
Ni, Co & Cr RECOVERY FROM NICKEL LATERITE LEACH LIQUORS BY BIOSORPTION USING IMMOBILISED ALGAL BIO-MASS: S. Agatzini-Leonardou, J.G. Zafiratos, Laboratory of Metallurgy, Department of Mining and Metallurgical Engineering, National Technical University of Athens, Athens, Greece
The objective of this work was to prove repeated loading capacities of physically stabilized algal biomass from Spirulina platensis and Chlorella sp. for the removal and recovery of cobalt, nickel and chromium from laterite heap leach liquors. The testwork was carried out on a bench scale in small columns and proved that it is possible to immobilize the biomass on the highly porous surface of pumice stone. Nickel and cobalt could be removed from pure aqueous solutions of each metal by cyanobacterial biomass of the species Spirulina platensis and Chlorella sp. immobilized on the mineral. Nickel and also cobalt biosorption was sensitive to the initial solution pH. The maximum amount of cobalt was retained on the biomass at pH 6.0, while nickel was removed most effectively at pH 5.5. Repeated loading capacities of physically stabilized algal biomass from Spirulina platensis and Chlorella sp. were proven effective for the removal and recovery of cobalt and nickel from solutions resembling diluted laterite heap leach liquors. A lifetime of at least 5 loading cycles of the immobilized biosorbent material was obtained. The loading capacity of the immobilized biomass for nickel was comparatively lower than the capacity of the same biomass for cobalt. Cobalt recoveries up to 80% were achieved after 5 loading cycles under unoptimized conditions whereas nickel was removed completely (100%) under the same conditions. The described process might be applicable to recover cobalt from diluted laterite heap leach solutions.
| Search | Technical Program Contents | 1997 Annual Meeting Page | TMS Meetings Page | TMS OnLine |
|---|