 |
49 (11) (1997), pp. 31-37. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
 |
49 (11) (1997), pp. 31-37. JOM is a publication of The Minerals, Metals & Materials Society |
|---|
| CONTENTS |
|---|
|
|
To date, much of the research has focused on the high-performance lightweight needs of the aerospace industry, where the unique needs of the defense and advanced research organizations render cost a minor factor and reductions in structural weight are affected by reducing the alloy density and increasing its modulus. New and expensive 2224 extrusion and 2324 plate, which combine high damage tolerance with relatively high strength, have been developed to replace the current 7000 series aluminum alloys for lower wing panel/spar cap aircraft applications.5
The common material candidates are Al, Li, Mg, and Ti alloy composites as parts producers aim at improving the limiting properties of the conventional aluminum alloys for weight reduction and elevated temperature (e.g., for engine components and highly loaded engines), wear resistance (e.g., for brake discs, drums, and cylinder blocks), modulus of elasticity (e.g., for calipers), and increased length (e.g., for driveshafts).
For applications in the automotive, transportation, construction, and leisure industries, affordable cost is also an essential factor. Apart from the emerging economical processing techniques that combine quality and ease of operations,6-8 researchers are, at the same time, turning to particulate-reinforced aluminum-metal matrix composites (AMCs) because of their relatively low cost and isotropic properties4,6,9 especially in those applications not requiring extreme loading or thermal conditions (e.g., automotive components). Also, the processing problems and commercial difficulties associated with continuously reinforced AMCs9,10 are contributory to the recent interest in their particulate counterparts; the use of aluminum alloys for the matrix is preferred because of its comparative advantages,11 including low cost ($1.5/kg) and ease of handling.
The particulate reinforcements have been classified as the by-products from other technologies (e.g., SiC, SiO2, Al2O3, aluminosilicates, graphite, and fly-ash) and are readily available or are naturally renewable at affordable cost (e.g., coconut shell char, mica, palm-kernel shell char, and zircon). Further, the potential nature of these filler materials is attractive. For example, SiC has good thermal and chemical stability, both during synthesis and under severe service conditions, strength, cost (about $13/kg for the particulate), and availability. The specific applications of these composites include engine blocks, pistons, brake-system components, seals, solid lubricants, wear- and abrasion-resistant structures, electromechanic contacts, and chassis components.
In the past five years, emerging synthesis techniques have improved the array of fabrication methods for particulate composites with differing reproducible structures and properties.9,12 The most cost-effective methods are currently casting 4,13 and powder metallurgy (P/M).9,14 Since the introduction of AMCs in commercially available quantities, both methods, as well as new techniques, are refocusing directly on net-shape products.6,8,15-18 Most of the work in this emerging front has used Al-Si alloys as the base material, most commonly the hypereutectic, hypoeutectic, and A356 alloys.19-22 A brief background on these processes in provided in the sidebar.
The Al-Si alloy is a well-known casting alloy with high wear resistance, low thermal-expansion coefficient, good corrosion resistance, and improved mechanical properties at a wide range of temperatures. These improvements are significant not only when the silicon phase undergoes structural modifications or a grain refinement is achieved through solute additions, mechanical pressure, or subfusion consolidation measures, but also when the silicon particles are finely distributed in the matrix phase. With improved fineness and distribution of the silicon particles, the machinability of the hypereutectic-alloy composites are accentuated.
These properties led to the application of Al-Si alloys in the automotive industry, especially for cylinder blocks, cylinder heads, pistons, and valve lifters. They are extensively used in tribological ends, but their poor resistance to seizure makes them vulnerable under poor lubricating conditions. The addition of ceramic particulate to these alloys increases stiffness, high-temperature strength, and wear resistance at ambient temperature44 while the use of graphite (or carbonaceous precursors) and mica lend it to reduced friction applications such as bearings, solid lubricants, and braking components.9,45,46
| Table I. The Composition of Common Al-Si Casting Alloys* | |||||||
|---|---|---|---|---|---|---|---|
| Alloy | Fabricationý Method |
Element (wt.%) | |||||
| Si | Cu | Mg | Fe | Zn | Others | ||
| 350 Series | S/PM | 5-7 | <0.2-1.25 | 0.35-0.55 | <0.06-<0.2 | <0.1-<0.35 | — |
| 380 Series | D | 8.5-11.0 | 2.0-3.5 | <0.1-<0.3 | <1.3 | <3.0 | <0.3 Sn |
| 390 Series | D | <7.5 | 4.5 | 0.55 | <1.3 | <0.1 | <0.1 Mg |
| 400 Series | S/PM/D | 5.25-12.0 | <0.1-<0.3 | <0.05-<0.1 | <0.8-<2.0 | <0.5 | — |
| * The remainder of the composition is aluminum and impurities. S=Sand casting; PM=Permanent mold casting; D=Pressure die casting. |
|||||||
In the Al-Si equilibrium diagram, the binary eutectic or hypoeutectic alloys are characterized by good castability and corrosion resistance, while the hypereutectic alloys, such as 390 and 393, containing 15-25% silicon exhibit excellent wear resistance and low thermal-expansion coefficients (CTE); their machinability is improved as the silicon particles become finer and more evenly distributed. Strengthening these monolithic alloys is achieved by small additions of elements such as magnesium, copper, and nickel, which also bring about changes in other properties. For example, in hypoeutectic alloys, silicon provides good casting properties, and copper improves tensile strength, machinability, and thermal conductivity at the expense of a reduction in ductility and corrosion resistance. The 332 alloy, which contains higher silicon (approximately 9.5 wt.% silicon) and copper levels, is used in internal-combustion engines because of its thermal stability and lower CTE.
| PROCESSING ALUMINUM-ALLOY COMPOSITES: A BACKGROUND |
|---|
| Several investigators have reviewed the synthesis of MMCs6,28,24 and, especially, AMCs.6,23-25 Traditional molten processing (MP) involves a variety of methods, including mixing/vortex, infiltration, and rheocasting; some of these MP routes are currently being extended to a secondary processing stage such as extrusion. Methods other than the MP route include P/M, spray atomization/codeposition (SD), and in-situ production.
Mixing/Vortex In the mixing/vortex method, the pretreated and prepared filler phase is introduced in a continuously stirred molten matrix and then cast. The use of an inert atmosphere or vacuum other than air is essential to avoid the entrapment of gases. Mixing can be affected ultrasonically or by reciprocating rods, centrifuging, or zero-gravity processing that utilizes an ultrahigh vacuum and high temperatures for long periods of time. A method of inertial injection has been developed for this process.7 Difficulties, such as the segregation/settling of secondary phases in the matrix, agglomeration of ceramic particulate, particulate fracture during agitation, and extensive interfacial reactions, are often encountered. The DURAL process, which incorporates SiC and Al2O3 particles into molten aluminum, makes use of this method.8 Infiltration In infiltration, the molten metal penetrates a pretreated, formed, and prepared particulate bed or preform with pressure or without pressure (pressureless infiltration). In the latter case, however, the molten alloy infiltrates the reinforcement by percolation. This method is normally carried out in air, inert gas, or evacuated atmosphere. Mortensen et al.26 have associated this technique with such disadvantages as reinforcement-reinforcement contact, structural distortion of the preform, large grain size, and undesirable interfacial reactions that culminate into microstructural inhomogeneity. The first commercial application was the fabrication of aluminum-alloy diesel pistons containing alumina short fibers by Toyota Motor Corporation.27,28,29 Squeeze casting was the primary manufacturing mode. Aluminum-alloy melt was poured into a porous alumina short-fiber preform inserted into a preheated die, and a squeeze pressure was applied in a hydraulic press. The composite aluminum pistons possessed better performance attributes than the unreinforced ones. The Lanxide process, which is a melt oxidation process, is another infiltration route in the synthesis of AMCs. In fact, Lanxide and Alcan have jointly produced an Al2O3/Al alloy composite of exceptional low erosive wear rate30 through this process. It involves the infiltration of a final-product-shape ceramic preform by a molten alloy. The preform is normally formed by pressing, slip casting, joining, or injection molding. In air or under a preferred gas, the molten alloy slips through the preform and oxidizes or chemically reacts with the preform material. The final composite phases consist of the oxidation (or reaction) products and the remaining matrix material. By this method, a dense composite shape is usually achieved. RheocastingRheocasting (or compocasting) permits the introduction of the pretreated particulate or short fibers into the solidifying, highly viscous, and thixotropic dendritic slurry of the molten matrix by agitation. This mechanically entraps the ceramic reinforcements and prevents any form of segregation. Continued stirring then reduces the viscous mass to low-viscous, fine, nondendritic slurry. This results in a mutual interaction between the matrix melt and the filler phase, which enhances wetting and bonding between the two phases. Pressure is usually used to effect a sound casting,31,32 especially when the volume fraction (Vf) of the particulate material is greater than 0.3 or 0.15, in the case of short fibers. This is because the composite viscosities increase as Vf increases, which is limiting at lower volume fractions. Fiber damage/degradation due to vigorous agitation is another difficulty. Owing to these effects, rheocasting lends itself only to particulate composites with a very low Vf of low to medium density particulates.32,33 A fundamental characteristic of this technique is that the matrix alloy is isothermally held within the freezing range of the alloy and, together with the reinforcement, is mechanically stirred. Stirring and agitation help to break the solid phases into smaller forms, releasing any particulate clusters that also break down in the process. New particle-matrix bonding can then take place, through which particulate agglomeration and gravity-induced settling is eliminated. Powder MetallurgyP/M is used in the synthesis of both AMCs and ceramic-matrix composites through the relatively low-cost methods of single compaction, double compaction, and mechanical deformation following hot pressing as well as through high-cost hydrostatic and isostatic compaction, hot dynamic compaction, or explosive compaction methods. P/M involves the blending of well-characterized matrix powders and discontinuous reinforcement, compaction at ambient or hot conditions, degassing, and consolidation. In these solid-state techniques, subfusion temperature regimes are normally attained in consolidation for optimum results. Depending on the morphology of the reinforcement or the desirable properties, further processing by mechanical-deformation mechanisms are applied. Alcoa, DWA Composite Specialties, Ceracon, and the Advanced Composite Materials Corporation are using this method in some of their commercial operations.17,34,35 Through the cold compaction of a pretreated elemental matrix blend and particulate mix followed by optimum consolidation conditions by sintering, highly wear-resistant zircon/Al-Si composites have been recently reported by J.U. Ejiofor et al.36 Another recent study has also produced wear-resistant char/Al-Si-Mg composites with 0.02 Vf via reaction sintering.9 Inal and coworkers6 have used the explosive compaction to fabricate SiC-reinforced 7093 AMCs. During explosive consolidation, a strong shock-hardening behavior of the matrix alloy was found. Similar to P/M is the solid-phase synthesis of particulate AMCs by rapidly quenching the metal powders and fine ceramic particulate using high (mechanical or electrical) energy sources to consolidate the mixture in as short a time as possible. It is a high-energy, high-rate process. The short time at temperature benefits phase-transformation control and reduces the chance of a degeneration into coarse microstructure. The P/M process has been successfully applied in the manufacture of both Al-SiC and SiC/Ti3Al + Nb composites.37 Spray Atomization/CodepositionSD is gaining recognition in the synthesis of discontinuously reinforced MMCs.4,38-40 The process involves the incorporation of fine ceramic particulates in inert-gas-atomized droplets of the molten matrix such that the matrix contains both liquid and solid phases. The matrix material is usually finely dispersed in droplets by the high-velocity spray of the inert-gas jets. The materials and structural-design advantage of this process is that desired multiphase matrix materials or discontinuous reinforcement, while entrained in a gas jet, could be incorporated at a localized portion. Unwanted reactions are avoided because the contact time and the thermal exposure between the particulate and the partially solidified matrix phases are reduced. The Osprey deposition technique, a two-phase process that has found application in Alcan productions, is a rare technique.41 Here, the molten-metal-alloy matrix on which the reinforcement particulate is injected is atomized by spray jets of inert gas. The solid mixture can then be collected on a nonwetting substrate in the form of a consolidated, reinforced composite mass. A recent report on this practice39 has reaffirmed that spray-atomized products are not only free from microsegregation and low in gas contents, but also exhibit certain characteristics that are associated with rapid solidification. Other process benefits over ingot metallurgy include low capital costs (less equipment required), low operating costs (low energy consumption and high material yields), and low overhead costs (less stock and work-in-progress). Al-Si alloy extrusion billets with excellent dimensional tolerances were recently produced via the Osprey deposition technique.39 In-Situ ProductionAnother growing route that is attracting a number of researchers is the in-situ production of reinforcement particles in the matrix.6,15,42,43 Many of these researchers have reported intrinsic uniformity in the distribution of the reinforcing phases. Also, many processes can be used to produce these in-situ reinforcements, including the formation of compounds and their decompositions, redox reactions, phase changes, nucleation, and recrystallization. These processes usually produce periodic microstructural features that account for the uniformly distributed phases achieved. In this production route, particles are obtained in the solvent (which can exist in any three states of matter) due to chemical reaction or diffusion, which usually occur under isothermal conditions. Chen and Chung have applied a new stir-casting technique to fabricate in-situ AMCs containing approximately 5 vol.% TiAl3 particles.6 The method involved the stir casting in air of a slurry consisting of molten aluminum, TiO2 particles, and Na3AlF6 particles. According to their report, the composites demonstrated higher tensile strength and ductility than the SiC-reinforced aluminum composites. |
This flexibity in component casting and heat treatment, the ability to refine the melt grains and modify the structures, and their applications properties are among the factors that make Al-Si alloys attractive. Various methods and designs are now being used8,9,38,39,55 to harness and improve on these advantages in the synthesis of composite materials.
This observation calls for critical research investigations into the potentials of this technique in the synthesis of particulate Al-Si MMCs. Unlike short-fiber preforms, the preparation of particulate preforms appears simple and, therefore, has not attracted critical scientific investigations. To infiltrate particulate preforms with little or no pressure requires a superheated melt alloy with a composition of wide-freezing range in order to avoid a high viscous flow until the desired melt-preform interactions and consolidation are achieved. Because of the high fluidity imparted by additions of silicon to aluminum, hypereutectic alloys are the best candidates for this method. Although coarse silicon particles or a-Al phases may predominate the microstructure and impair some useful properties, treatments such as grain refinement, structural modifications by elemental or flux additions, or a subsequent processing by deformation would tremendously improve both mechanical and tribological properties.48,49,58
Pressure infiltration, on the other hand, is expected to produce composites of higher quality, especially when deformation processes are designed into the manufacture. Possible disadvantages may include preform failure and particulate-to-particulate contact.26 This can be checked by applying optimum pressure vis-a-vis both to the integrity of the preform and the thermodynamic processes in the molten mix, selecting and pretreating those particulates that will form networks (through phase formations) on contact with the alloy melt, incorporating the fillers at high Vf, and maintaining the mold temperature at a level halfway in the freezing range during the application of pressure. A related principle (the melt-oxidation process) has been used by Lanxide and Alcan30 in producing Al2O3/Al composites that have low erosive wear rates.
Chen and Chung59 have reported a new composite network comprising interpenetrating networks of silicon (69 vol.%) and aluminum (31 vol.%) fabricated by liquid infiltration of Al-30Si-1Mg into a 50 vol.% silicon-particle preform. The process involved vacuum infiltration of the liquid alloy under an argon pressure of 41 MPa at 900°C, and the composite structure appeared to be characterized only for CTE. Information on the other properties and the microstructural and phase analyses of the composites were not provided. Such information could have explained the phases and internal features, including the continuity of the network and the system, and shed light on the optimum Vf of the preform necessary to yield a good particle-matrix network. It would also be possible to design the desired property level for its end use (such as thermal properties for electronic packaging) into the composite composition. In this composite system, such a design has the potential of changing the classification of the product from an MMC to a silicon-based composite network. However, this composite possessed a low CTE of 7.2 X 10-6°C-1, comparable to those of conventional AMCs containing more than 70 vol.% particles such as SiC or AlN.60 This makes it attractive for electronic packaging. The P/M particulate composite (60 wt.% aluminum and 40 wt.% silicon) made by Sumitomo Electric Industries Ltd., Japan, had only an ambient temperature CTE of 13 X 10-6°C-1 and thermal conductivity of 165 W.m-1K-1.61 The Chen and Chung method shows that this network was formed in-situ and not by the infiltrating action; such a structure is expected to lead to an increased mechanical strength due to its interlocking nature, although it has a disadvantage of reduced ductility due to stress build-up associated with such structures.
Earlier work by Yang and Chung fabricated (SiC)p/Al-12Si, (Al2O3)p/Al-12Si, and graphite-reinforced aluminum composites using a similar technique55 that combines vacuum infiltration, infiltration under an inert gas, and squeeze-casting principles. No information on property characterization was provided. It is noted that a SiC particulate preform with a high Vf particulate is completely infiltrated by the aluminum melt at low pressure (unlike an alumina preform), and no porosity was observed in the SiC-reinforced composites. This is because (SiC)p wets the aluminum melt more than (Al2O3)p and is more reactive with it, suggesting that the Vf of SiC particles should be much higher than that of Al2O3 particles in AMCs with the same pressure and amount of matrix. Attempts to infiltrate soft-graphite particle preforms resulted in damaged and squeezed particles by the metallostatic head as compared to graphite-flake preforms. An inference from this behavior is that density, strength, and deformability of the filler-phase particles and preforms are independently critical to a homogeneous cast-composite structure.
This inference explains the ability of the porous alumina short-fiber preform55 used by Toyota Motor Corporation to achieve a homogeneous structure. The micrograph of alumina dispersed in aluminum in Figure 1 reveals that infiltration of the particulate preform containing particles of wide particle-size distribution was difficult, suggesting that more pressure than that of relatively uniform particle-size distribution is required. When the fine-size particles sit in the voids between the large-size particles, the available spaces become narrower or even closed, making penetration difficult.
An important advantage of this combination of principles is that the finer control of pressure under inert gas (over the use of the hydraulic press) makes it possible to achieve a reduced rate of pressure increases. This, in turn, reduces the compression on the preforms. Also, the higher temperature maintained during infiltration makes it possible for the melt to penetrate the pores, leading to a fully dense structure. With this, the method can be applied to porous short-fiber-reinforced AMCs (Figure 2). A redesign of the hot-isostatic-press apparatus is necessary to reduce costs and achieve a faster cooling rate. In addition, melt treatments can be used to improve the microstructure of the final composites.52 A full characterization of the composite structures is, however, required in order to compare the technique to similar well-known casting methods.
 |
| Figure 1. A micrograph of aluminum reinforced with Al2O3. Penetration of the molten metal was difficult because of wide particle-size distribution of the reinforcement.55 |
 |
| Figure 2. A scanning electron micrograph of SiC whisker-reinforced aluminum (Vf = 0.15).55 |
The Duralcan impeller-mixing process (DuralcamTM owned by Alcan Aluminum) is a major development.67 While other mixing methods require the additions of surface-active elements to the alloy matrix or the use of metal-coated ceramic reinforcements at low Vf (<10 vol.%), the Duralcan process, which can be applied to conventional aluminum alloys, uses uncoated ceramic particles greater than about 10-12 mm and produces reinforcement levels of up to 25 Vf. Nevertheless, the problems of matrix-particulate reactivity and particle-segregation effects are somewhat encountered. The Duralcan composite is now being commercially produced in batches of up to 6,800 kg and cast as pig or direct-chill ingot. Hydro Aluminum AS has derived comparable composites via a mixing method,68 and Comalco recently introduced Comral, which is 6061 reinforced with a spherical Al2O3-SiO2 particle.69
The vortex method seems attractive because of its ease of operation and relatively low cost, and the drawbacks are continuously being addressed by recent research activities. Published papers on the fabrication of AMCs by stir casting67,70,71-76 indicate that a requirement for a good stirring unit is to provide intimate contact while minimizing gas absorption. Figure 3 shows the schematic diagram of a mechanical stirring device developed for this purpose.76 This installation provides a sharp dose of separated powder particles to the required point of the melt preceded by the displacement caused by the impeller. This is achieved through a mechanism where the powder-supply tube rotates with the impeller, and, as a result, the powder carried by the flow of inert gas always penetrates the zone of the melt with the highest turbulence. Increased particle content increases melt viscosity such that it can become non-Newtonian, but increased shear rate at increasing temperatures appears to minimize the viscosity.71 Also, particles recovery and microstructural homogeneity in cast composites prepared with the use of a mechanical impeller have been found to depend on the stirring rate and length of time at temperature, matrix-alloy composition, physicochemical nature of the reinforcement, feed time and feed rate, and crucible-to-impeller diameter ratio.70 Optimization of these variables is relevant to reproducible microstructure and properties.
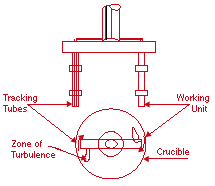 |
| Figure 3. A schematic of the vortex method showing the position of the tracking tubes on the working units.76 |
Available literature indicates that many scientific studies on this system make use of composites fabricated through the vortex method.8,18,19,64,72-75 Reproducible properties in cast-particulate composites are primarily dictated by the uniformity of the second-phase dispersion in the matrix. The distribution is controlled by solidification and can be later modified during secondary processing. Similarly, wetting of the dispersoid with the matrix is important in obtaining better strength properties in the composites; this is normally achieved by subjecting the dispersoid to mechanical, thermal, or physicochemical adaptation treatments. The particles can be treated in a vibrator, in which case, gradual abrasion and removal of the surface layer of particles takes place.7 Subsequent cleaning removes the dust, and the particle surface is removed. Thermal adaptation can include the heating of the reinforcing phase up to a temperature near that of the melt prior to introduction into the melt. Also, conditions at the dispersoid/matrix interface have to be controlled to optimize the bond strength. Surface treatments of SiCp using acetone and ultrasound simulta neously followed by strong heating of the particles have successfully removed adsorbed gases from the particle surface, leading to a uniform particle dispersion in the mechanically stirred A356 matrix melt containing 15 wt.% SiCp.72 With gas and shrinkage porosities observed in the composite castings, an addition of 3 wt.% magnesium in the matrix yielded an ultimate tensile strength (UTS) of 110-130 MPa and 2% elongation.
In another development,73 wear at ambient temperature in a Duralcan 20 vol.% SiCp/Al-7Si composite occurred by the breakage of silicon particles while the SiC remained intact. At 200°C, wear was controlled by subsurface deformation of the matrix, with both silicon and SiC fragmenting near the sliding surface. J.U. Ejiofor and R.G. Reddy have recently reported similar mechanisms of wear.11 Specific wear increased by approximately 140 m3/m.N X 10-13 from ambient-temperature value.
Another investigation by Ramani et al.74 observed a linear relationship between porosity in 20 vol.% and 30 vol.% SiCp/6061 composites and the volume percentage of the dispersoids. The SiCp was preheated at 750°C for two hours prior to introduction into the mechanically stirred melt and was subsequently cast in permanent molds. Uniform distribution of the reinforcements was achieved, and shrinkage and gas porosities were observed as the amount of SiCp increased. A total avoidance of porosity in this technique is difficult because the lower thermal conductivity of ceramic reinforcements requires them to be pushed to the solidifying front of a freezing melt such that shrinkage porosities appear around the particulate as the matrix shrinks in solidification. Also, as magnesium is surface active, it effectively reduces interfacial energies only with an optimum amount of reinforcements (i.e., free surface); otherwise, both gas (due to air layer) and shrinkage porosities will result.
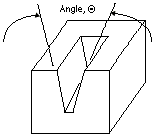 |
| Figure 4. A schematic of a wedge mold. A wedge mold produces segregation-free, high-Vf Al-Si composites.18 |
These results agree with those of Plate,77 who reports an increase in the UTS of aluminum and a decrease in the tensile strength of Al-12Si with the addition of alumina particles. Also, the wear resistance of Al2O3/Al-16Si is higher than that of Al2O3/Al-11.8Si, which, in turn, is higher than that of Al2O3/Al at 1- 5 wt.% Al2O3. Shrivanath et al.78 have reported similar results in an earlier work with slightly varied experimental conditions.
 |
| Figure 5. A typical micrograph of a 3 wt.% graphite/LM 13 composite.80 |
The most investigated matrices have been LM 13 (Al-11.0Si-1.5Ni-1.0Cu-1.0Mg-0.8Fe-0.5Mn) and LM 30 (Al-17.0Si-4.5Cu-0.5Mg-0.1Ni-0.3Fe-0.1Mn). LM 13 is a standard piston alloy, whereas, LM 30 has a potential for use as cylinder liners in internal-combustion engines in place of the heavier cast iron.
The major difficulty in the preparation of cast graphite/aluminum-alloy composites appears to stem from nonwetting of graphite by aluminum melts. The contact angle of aluminum with graphite is about 155°C and is reported to remain unwettable at temperatures between the melting point of aluminum and 1,080°C.82 The major interest by scientists in the molten processing of this class of composites is, therefore, geared toward achieving good matrix-graphite wetting. The incorporation of coated graphite particles in a vortex through gas injection or with the aid of ultrasonic stirring and the addition of interfacial active elements83,84 has been reported to yield good results, although this is associated with high indirect costs.
A simple and inexpensive vortex method has dispersed uncoated but heat-treated graphite particles into the LM13 melts at a temperature above the liquidus.80 It was possible to disperse only 3 wt.% graphite, with a percentage recovery in the casting of 98%. No pores or voids are visible at the graphite-alloy interface, indicating good wetting (Figure 5). The tested mechanical properties of the composites containing up to 3 wt.% graphite are found to be adequate for several tribological applications (Figure 6).
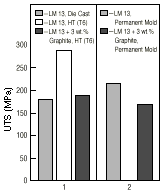 |
| Figure 6. A comparison of the ultimate tensile strength values of LM 13 composites. |
A homogeneous distribution of dispersoids in composites leads to improved results, and these were readily achieved in remelted composites.50,85 An Al-12Si-3Mg composite containing 3 wt.% graphite particles prepared by the vortex method was remelted and studied for microstructure and other properties.50 The temperature was maintained at 660°C in all the cases studied. Their BHN also improved by more than 25% with remelting and casting. Figure 7 presents some of these properties.
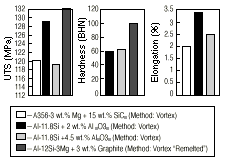 |
| Figure 7. A comparison of (a) ultimate tensile strength, (b) hardness, and (c) elongation of some Al-Si composites. |
Wang and Ajersch21 recently studied the solidification mechanism and structure of rheocast particulate Al-Si alloys filled with SiCp and Al2O3p. Their observation revealed that the primary a-phase does not nucleate on the reinforcing particles. Conversely, and substantiated by the reports of previous investigators90 who studied different composite systems, they also found that primary silicon nuclei form on the reinforcing particles. Later, Ribes and Suery91 investigated the age-hardening behavior of a compocast and pressure-squeezed-cast hypoeutectic Al-Si alloy containing SiC particles. While the aging response of the composites filled with unoxidized particles was accelerated and the extent of hardening relative to the unreinforced alloy was virtually unaffected, hardening was observed when oxidized particles were used.
The application of SD to Al-Si alloys has been minimally studied. Of the few studies that exist, spray-deposited Al-12Si/SiC and Al-4Si/TiB2 composites were produced and characterized for microstructure and mechanical properties.95 While the former composite system yielded an approximate 50% in-crease in strength because of good matrix-reinforcement bonding, poor bonding resulted in a negative effect on strength in the latter system. In both systems, however, uniform distribution of silicon particulate was achieved.
In-situ processing focuses more on the reactive nickel and titanium alloys and intermetallics,6,101-105 as well as on ceramic-oxide systems.106,107 Conversely, the in-situ formation of SiC and TiC via reactive infiltration of molten alloys Al-Si and Al-Ti into a carbon or graphite preform has been reported,108 and only the characterization of the reactions by microstructural analyses using x-ray and microscopic tools were reported.
R.G. Reddy earned his Ph.D. in metallurgical engineering from the University of Utah in 1980. He is the ACIPCO chair professor at the University of Alabama, Tuscaloosa. Dr. Reddy is a member of TMS.
For more information, contact R.G. Reddy, University of Alabama, A-129 Bevill, Tuscaloosa, Alabama 35487-0202; (205) 348-4246; fax (205) 348-2164; e-mail rreddy@coe.eng.ua.edu.
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|