
An Article from the June 2003 JOM: A Hypertext-Enhanced Article
 |
TMS
ONLINE | TMS
PUBLICATIONS | SITE
MAP An Article from the June 2003 JOM: A Hypertext-Enhanced Article |
|
|
|
|
S.A. David, S.S. Babu, and J.M. Vitek are with Oak Ridge National Laboratory.
|
Exploring traditional, innovative, and revolutionary issues in the minerals,
metals, and materials fields.
|
| OUR LATEST ISSUE |
|
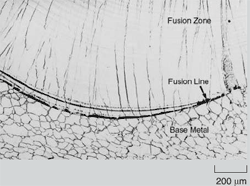
In welding, as the heat source interacts
with the material, the severity of thermal
excursions experienced by the material
varies from region to region, resulting
in three distinct regions in the weldment
(Figure 1). These are the fusion zone
(FZ), also known as the weld metal,
the heat-affected zone (HAZ), and the
unaffected base metal (BM). The FZ experiences melting and solidification,
and its microstructural characteristics
are the focus of this article.
The microstructure development in
the FZ depends on the solidification
behavior of the weld pool. The principles
of solidification control the size and
shape of the grains, segregation, and the
distribution of inclusions and porosity.
Solidification is also critical to the hot-cracking behavior of alloys. Sometimes,
it is convenient to consider the FZ as
a minicasting. Therefore, parameters
important in determining microstructures
in casting, such as growth rate (R), temperature gradient (G), undercooling
(DT), and alloy composition determine
the development of microstructures in
welds as well. Comprehensive reviews
of weld pool solidification based on
these parameters are available in the
literature.1,2
Most knowledge of weld pool solidification is derived from the extrapolation
of the knowledge of freezing of castings,
ingots, and single crystals at lower
thermal gradients and slower growth
rates.1–6 In addition, rapid solidification
theories have been extended to welds
solidified at very high cooling rates.7–14However, microstructure development
in the FZ is more complicated15,16because of physical processes that
occur due to the interaction of the heat
source with the metal during welding,
including re-melting, heat and fluid
flow, vaporization, dissolution of gasses,
solidification, subsequent solid-state
transformation, stresses, and distortion.
These processes and their interactions
profoundly affect weld pool solidification
and microstructure. In recent years,
phenomenological modeling of welding
processes has provided unprecedented
insight into understanding both the
welding process and the welded materials.
A variety of sophisticated models
that employ analytical and numerical
approaches are capable of describing
many physical processes that occur
during welding.15–25
During the past 15 years, significant
progress has been made in understanding
the solidification behavior of
the weld pool and the evolution of
microstructure in the FZ. The application
of computational thermodynamic
and kinetic tools has enhanced the
understanding of weld solidification
behavior of complex multi-component
systems. Advanced in-situ characterization
techniques have enabled the
characterization of phase formation and
non-equilibrium effects during weld
pool solidification. The use of model
alloy single crystals resulted in new
insight into the role of weld pool
geometry and dendrite growth selection
processes in the development of weld
microstructure. This overview will
address some of the current progress in
understanding weld pool solidification.
An important aspect of weld solidification is the dynamics of weld pool development and its steady-state geometry.
Weld pool shape is important in
the development of grain structure and
dendrite growth selection process.6, 26-29Thermal conditions in and near the
weld pool and the nature of the fluid
flow have been found to influence the
size and shape of the weld pool.16–18,24,25Significant advances have been made
in recent years to understand, in greater
detail, the dynamics of the heat and fluid
flow in the weld and the subsequent
development of the pool shape. To a
large extent, convective flow in the
weld pool determines weld penetration.
For arc-welding processes, convection
in the weld pool is mainly controlled
by buoyancy, electromagnetic forces,
and surface-tension forces. In actuality,
depending on the interplay between
various driving forces, the convective
flow could be simple or more complex
with a number of convective cells
operating within the weld pool, as
shown in Figure 2.
Recent theoretical developments
include the formulation of a free-surface
computational model to investigate
coupled conduction and convection
heat-transfer models to predict not only
weld pool geometry but also thermal
profiles to estimate thermal gradients
and cooling rates critical to determining
solidification structure.25 In addition
to computational models, neural net
models have been applied to predict
weld pool geometry.30 These models,
which are empirical in nature, are
useful when applied to complex welding
processes such as hybrid laser-arc
welding.30
Unlike in casting, during welding,
where the molten pool is moved through
the material, the growth rate and
temperature gradient vary considerably
across the weld pool. Geometrical
analyses have been developed that relate
welding speed to the actual growth rates
of the solid at various locations in the
weld pool.1,2,27
Along the fusion line the growth rate
is low while the temperature gradient
is steepest. As the weld centerline is
approached, the growth rate increases
while the temperature gradient decreases.
Consequently, the microstructure
that develops varies noticeably from
the edge to the centerline of the weld.
Most of these microstructural features
can be interpreted by considering
classical theories of nucleation and
growth.
In welds, weld pool solidification
often occurs without a nucleation barrier.
Therefore, no significant undercooling
of the liquid is required for nucleation
of the solid. Solidification occurs
spontaneously by epitaxial growth on
the partially melted grains. This is the
case during autogenous welding. In
certain welds, where filler metals are
used, inoculants and other grain-refining
techniques are used in much the same
way as they are in casting practices.
In addition, dynamic methods for
promoting nucleation such as weld-pool
stirring and arc oscillation have
been used to refine the weld metal
solidification structure.2 Although the
mechanisms of nucleation in weld metal
are reasonably well understood, not
much attention is given to modeling this
phenomenon. Often, weld solidification
models assume epitaxial growth and
for most of the cases the assumption
seems to be appropriate. However, to
describe the effects of inoculants, arc
oscillations, and weld pool stirring, heat
and mass transfer models18,24,25 have
to be coupled with either probabilistic
models such as cellular automata31–33or deterministic models using the
fundamental equations of nucleation as
described elsewhere.34
|
|
||
|
Figure 3. A scanning-electron micrograph showing the development of dendrites in a nickel-based superalloy single-crystal weld. |
Figure 4. An optical micrograph shows the change in dendrite morphology from cellular to dendritic as the growth velocity increases toward the center of spot weld (from bottom to top) after the spot weld arc is extinguished. |
|
|
|
During growth of the solid in the weld pool, the shape of the solid-liquid interface controls the development of microstructural features. The nature and the stability of the solid-liquid interface is mostly determined by the thermal and constitutional conditions (constitutional supercooling) that exist in the immediate vicinity of the interface.35,36 Depending on these conditions, the interface growth may occur by planar, cellular, or dendritic growth. Dendritic growth of the solid, with its multiple branches, is shown in Figure 3. Another example of changes in solidification morphology directly related to welding conditions is shown in Figure 4. This figure shows a spot weld on a nickel-based superalloy in which the morphology changes from cellular to dendritic as the growth velocity increases toward the center of the spot weld after the spot weld arc is extinguished. The micrograph also shows the elimination of a poorly aligned dendrite, which is discussed in greater detail later. The criterion for constitutional supercooling for plane front instability can be mathematically stated as:
plane front will be stable |
(1)
|
planar instability will occur |
(2)
|
where GL is the temperature gradient
in the liquid, R is the solidification
front growth rate, DTO is the equilibrium
solidification temperature range (at
composition CO), and DL is the solute
diffusion coefficient in liquid.
|
|
|
|
Figure 5. Epitaxial and columnar growth near the fusion line in an iridium alloy electron-beam weld. The figure also shows the grain-growth selection process of the grains from the fusion line. |
Solute distribution during weld
pool solidification is an important
phenomenon resulting in segregation
that can significantly affect weldability,
microstructure, and properties. Studies
extending different solidification models
to describe solute distribution during
weld solidification are summarized
elsewhere.2 In describing the solute
distribution under dendritic growth
conditions, consideration should be
given to redistribution at the dendrite
tip and in the interdendritic regions.
In welds, since the microstructures are
much finer in scale than in castings, the
contribution to the total tip undercooling
due to the curvature effect is significant.2 The effect of increased undercooling
at the dendrite tip would be to solidify
at a composition closer to the overall
composition and thus reduce the extent
of microsegregation. Dendrite tip
undercoolings in welds have been
estimated by measuring dendrite core
compositions for Al-Cu and Fe-Nb
systems after welding.38 For solute
distribution in the interdendritic regions
it may be sufficient to extend the
solidification models for microsegregation
in castings to welds. This can be
achieved by the Schiel equation39 or
modified Schiel equation that considers
the diffusion in the solid during
welding.38,40
As mentioned earlier, since solidification
of the weld metal proceeds spontaneously
by epitaxial growth of the partially
melted grains in the base metal, the FZ
grain structure is mainly determined by
the base metal grain structure and the
welding conditions.2 Crystallographic
effects will influence grain growth
by favoring growth along particular
crystallographic directions, namely the
easy growth directions.35,36,41 For cubic
metals, these easy directions are <100>.
Which of these <100> directions will be
selected, a fundamental question that is
important when welding single crystals,
will be addressed later. Conditions for
growth are optimum when one of the
easy growth directions coincides with
the heat-flow direction. Thus, among
the randomly oriented grains in a
polycrystalline specimen, those grains
that have one of their <100> crystallographic
axes closely aligned with
heat-flow direction will be favored.
Without additional nucleation, this will promote a columnar grain structure.
Figure 5 shows clearly the grain growth
selection process in an iridium alloy
weld. Under certain conditions it is
also possible to change the epitaxial
columnar growth to equiaxed growth
by inoculation or changing welding conditions.28,42,43
Studies on Fe-15Ni-15Cr single-crystal
welds carried out during the last
ten years have advanced significantly the
fundamental understanding of weld
pool solidification.27–29 These studies
have identified the effect of crystallography
on the development of FZ
microstructure. A geometrical model
has been developed that provides a
three-dimensional relationship between
travel speed, solidification velocity, and
dendrite growth velocity that predicts
stable dendrite growth directions as a
function of weld pool shape and weld
orientation. The regions of differently
oriented dendrites develop because
growth occurs along the preferred <100> growth directions, and the choice
of which growth direction will prevail
among the six possible variants is based
on the relation between weld pool shape
and dendrite orientation. The model’s
capability to predict microstructural
features in an Fe-15Ni-15Cr singlecrystal
electron beam weld made along
[100] on (001) plane is shown in
Figures 6a and 6b.
Recently, these basic concepts have
been extended to commercial nickel-based
superalloy single-crystal technology technology
used in jet and land-based turbine
engines.44–46 Unlike in Fe-15Ni-15Cr
single-crystal welds where the single
crystallinity of the weld was maintained,
nickel-based superalloys are extremely
prone to stray grain formation (as shown
in Figure 7). This phenomenon can be
attributed to constitutional supercooling46,47or dendrite fragmentation48 ahead
of the dendritic front that may nucleate
new grains. Recent studies suggest that
the constitutional supercooling may be
the controlling mechanism for straygrain formation.44,47
|
|
||||
| a | b | |||
|
Figure 6. (a) An Fe-15Cr-15Ni single-crystal electron-beam weld made along [100] direction on (001) plane, and (b) the calculated dendritic growth pattern for a similar weld orientation in (a). |
Figure 7. An optical micrograph of overlapping laser spot welds on PWA-1480 single-crystal nickel-based superalloy showing the formation of stray grains at the center of the weld. |
|||
|
|
||||
Because of the rapid cooling rates
encountered during welding, especially
during high-power-density processes
such as electron and laser-beam welding,
it is not uncommon to observe
nonequilibrium solidification effects.
Most nonequilibrium features in welding
can be associated with two phenomena
that take place as the solidification
growth velocities increase. First, the
partitioning of solute between solid and
liquid, described by the partitioning
coefficient k (= solid composition/liquid
composition, both at the solid/liquid
interface), is affected by growth rate such
that, as the growth velocity increases, k deviates from the equilibrium value and
approaches a value of 1. Second, high
growth velocities can lead to a change
in the solidification mode and result in
nonequilibrium phase formation. It is
noteworthy that these phenomena are
closely interrelated.
As discussed earlier, the solidification
morphology also changes with growth
velocity and is influenced by the extent
of solute partitioning and the phase that
forms. In this section, nonequilibrium
solute partitioning will be addressed, but
even equilibrium solute partitioning can
lead to nonequilibrium phase formation
because of residual microsegregation;
this can be evaluated by the Scheil
equation and its variants.
|
|
||
| a | b | |
|
Figure 8. Photomicrographs of high-speed laser welds showing (a) fully ferritic microstructure in type-312 stainless steel with negligible secondary austenite formation and (b) nonequilibrium austenitic microstructure in type-308 stainless steel without any ferrite formation. |
||
|
|
||
Theories have been developed to
relate the degree of partitioning to the
growth rate.14 For high growth rates
that may be prevalent during welding,
reduced solute partitioning resulting
from a change in k can lead to a variety
of effects including morphological
changes to plane front solidification, changes in the solidification phase,
and less segregation in the weld
microstructure. An example is shown
in Figure 8a, where an autogenous
laser weld was made on a 312 stainless-steel
weld overlay pad. The laser-weld
microstructure is fully ferritic, which
reflects the fact that minimal partitioning
during solidification prevented
secondary austenite formation, as found
in the weld overlay. In this case, the
rapid cooling conditions during laser
welding also prevented solid-state
transformation of the solidified ferrite to austenite.
Numerous examples of nonequilibrium
solidification in austenitic stainless
steels have been documented over the
years.8–11,49 An example is shown in
Figure 8b. In this case, the micrograph
is of an autogenous laser weld on a
308 stainless-steel weld overlay. The
base material (weld overlay), shown
on the left, shows the typical weld
microstructure in this material consisting
of austenite and residual ferrite.
This is produced by primary ferrite
solidification followed by secondary
austenite solidification and ferrite
transformation to austenite during
solid-state cooling. The laser-weld
microstructure is completely different.
It is a fully austenitic microstructure produced by nonequilibrium primary austenite solidification.
Another example of nonequilibrium
solidification in a low-alloy steel is
presented in the section on in-situ
observations. It is also noteworthy that
the laser-welded microstructure does
not show any dendritic structure;
this is another example of the solidification
morphology changing to planar
solidification at high growth rates.
Extremely high growth rates are not
necessary to produce nonequilibrium
solidification. A series of experiments
in which welds were made across
dissimilar stainless steels showedthat nonequilibrium solidification can be found even under less extreme
solidification conditions.50 Current
research focuses on the quantitative
prediction of these transitions from
equilibrium to nonequilibrium conditions
by numerical modeling of
weld solidification in multicomponentalloys.
In addition to heat and fluid-flow
models used for welding, additional
modeling techniques are now available
that can help describe the phase
evolution during weld solidification.
Foremost among these are computational
thermodynamic models for
multicomponent systems that can predict
the primary solidification phases, the
solidification phases that may form as
a result of solute partitioning during
solidification, and the stability of these
phases as the weldments are cooled to
room temperature. For example, one
such program, ThermoCalc,51 has been
used to calculate a phase diagram for
a hypothetical Fe-20Cr-8Ni-xN (wt.%)
alloy as a function of temperature and
chromium content for two different
nitrogen concentrations, x = 0.01% and
x = 0.1% (Figure 9a and Figure 9b, respectively).
The plots show that at 20% chromium,
for both 0.01% nitrogen and 0.1%
nitrogen, the primary solidification will
occur by d-ferrite. However, the phase
stability following solidification is quite
different. In the case of the low-nitrogen
stainless steel, at 800°C, a mixture of
ferrite and austenite is expected while a
fully austenitic structure is predicted for
the high-nitrogen alloy in equilibrium at
the same temperature. Such calculations
are simple and can be used to identify
the effect of alloy composition on the
phase stability during and after weld
solidification. Perhaps the greatest
benefit that results from these models is
that the calculations can be performed
easily for complex multicomponent
systems with ten or more constituents.
Kinetics models based on diffusion-controlled
growth can be integrated with
computational thermodynamics models
to provide valuable information on the
time evolution of the microstructure.52 For example, in the case of welding,
calculations can be made to identify
the effect of cooling rate on the final
microstructure.
Such calculations were made for the
two Fe-20Cr-8Ni-xN alloys described
above. The calculations assumed a
half-dendrite arm spacing of 100 µm
and a cooling rate of 10 Ks–1. The model
considered a peritectic solidification
mode, with primary ferrite formation
and secondary austenite formation at
the ferrite/liquid interface. The results
of the calculations are shown in Figure
9c and Figure 9d, where the phase fractions
are plotted versus time. In the case of
the high-nitrogen welds, the austenite
growth into ferrite phase was found
to increase rapidly after ~35 s. Thus,
the diffusion-controlled growth models
allow the calculation of the amount
of d-ferrite that may be retained after
solidification and the description of
the weld microstructure evolution in
stainless steels to a certain extent.
These calculations can be repeated
for different weld cooling rates and
dendrite arm spacings to evaluate the
effect of welding process parameters
on the microstructure.
As noted in the previous section,
nonequilibrium solidification may take
place at higher cooling rates and
solidification growth rates. Recent
advances in interface-response function
models53 can be used to evaluate the
phase selection during solidification in
multicomponent steels by coupling them
with computational thermodynamic
software. The interface-response function
model evaluates the dendrite tip
radius, tip temperature, and partition
coefficients as a function of interface
velocity for various competing phases and determines which solidification
phase is kinetically favored. The next
step in the modeling of weld solidification
is to couple computational thermodynamic,
diffusion-controlled growth
models, crystallographic geometry
models,27 and cellular automata54 models
to depict the fine details of microstructure
morphology as a function of composition and welding process parameters.
|
|
||
| a | b | |
| c | d | |
|
Figure 9. Quasi-binary diagrams showing liquid, austenite, and d-ferrite phase regions in Fe-Cr-Ni alloy systems with (a) 0.01 wt.% nitrogen and (b) 0.1 wt.% nitrogen. The calculated variation of phase fraction as a function of cooling time from 1,750 K using a diffusion-controlled growth model for Fe-Cr-Ni alloy systems with (c) 0.01 wt.% nitrogen and (d) 0.1 wt.% nitrogen. |
||
|
|
||
Modeling activities must be accompanied
by careful experimental measurements
in order to validate the models.
Traditionally, the evaluations of models
have been made by post-weld characterization
of solidification microstructures
using optical microscopy and analytical
electron microscopy. However, interpretation
of weld behavior by examination
of welds at room temperature is often
incomplete and complicated by phase
transformations that take place upon
cooling. There is a growing need to
monitor solidification microstructure
in-situ during weld cooling. Many
techniques are currently available to
observe the weld solidification features
in-situ, including high-speed, high-resolution
photography on real materials55 or on metal analog transparent
systems,37 and time-resolved x-ray
diffraction (TRXRD) with synchrotron
radiation.56
Recent results from metal analog
transparent systems, combined with
detailed numerical heat transfer models
and solidification theories, led to the
identification and analysis of instabilities
at the liquid-solid interface while
welding at high speeds.37 Additional
work has focused on nonequilibrium
phase selection during weld solidification
in an Fe-C-Al-Mn steel by means of
in-situ observations using the TRXRD
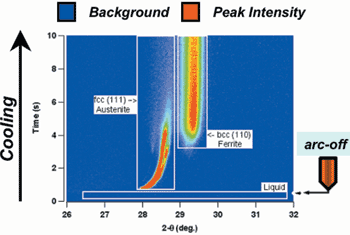
technique.57,58 In this research, the
equilibrium primary solidification phase
is d-ferrite and this was confirmed by
TRXRD measurements on slowly cooled
spot welds. However, under rapid
cooling conditions, the TRXRD measurements
showed the formation of
primary austenite (Figure 10). Research
in stainless steels has shown that it is
possible to form nonequilibrium primary
austenite under rapid solidification
conditions but this is the first time such
a phenomenon was observed in a
low-alloy steel. In these steels, in-situ
measurements are particularly valuable
since behavior at elevated temperatures
is masked by subsequent solid-state
transformation of ferrite to austenite and
austenite to martensite. Time-resolved
x-ray diffraction measurements have
proven to be ideal for identifying
competing phase-transformation mechanisms
under nonequilibrium weldcooling
conditions. This technique has
been applied to other alloy systems and
exciting new insight into issues relating
to weld solidification issues is being
achieved.59
|
|
|
|
Figure 10. An image representation of time-resolved x-ray diffraction data that shows the formation of primary austenite (fcc) from liquid during rapid cooling. |
This research is sponsored by the Division of Materials Sciences and Engineering, Office of Basic Energy Sciences, U.S. Department of Energy, under Contract DE-AC05-00OR22725 with UT-Battelle, LLC.
References
1. W.F. Savage, Welding World, 18 (1980), p. 89.
2. S.A. David and J.M. Vitek, Inter. Mater. Review, 34 (5) (1989), p. 213.
3. G.J. Davies and J.G. Garland, Inter. Mater. Review, 20 (1975), p. 83.
4. F. Matsuda, T. Hashimoto, and T. Senda, Trans. Natl.
Res. Inst. Met. (JPN), 11 (1) (1969), p. 83.
5. K.E. Easterling, Introduction to Physical Metallurgy
of Welding (London: Butterworths, 1983).
6. S. Kou, Welding Metallurgy, Second edition (New
York: John Wiley & Sons, Inc., 2002).
7. R. Mehrabian, Inter. Metall. Review, 27 (1982), p. 185.
8. J.M. Vitek, A. DasGupta, and S.A. David, Metall.
Trans., 14A (1983), p. 1833.
9. S.A. David, J.M. Vitek, and T.L. Hebble, Weld J., 66 (1987), p. 289s.
10. S. Katayama and A. Matsunawa, Proc. of Int’l
Congress on Application of Lasers and Electro Optics, vol. 4 (Boston, MA: Laser Institute of America,
1984), p. 60.
11. J.W. Elmer, S.M. Allen, and T.W. Eager, Metall.
Trans. 20A (1989), p. 2117.
12. R. Trivedi and W. Kurz, Acta Metall. 34 (1986),
p. 1663.
13. W.J. Boettinger and S.R. Coriell, Mater. Sci. Eng., 65 (1984), p. 27.
14. M.J. Aziz, J. Appl. Phys., 53 (1982), p. 1158.
15. S.A. David and T. DebRoy, Science, 257 (1992),
p. 497.
16. T. DebRoy and S.A. David, Reviews of Modern
Physics, 67 (1) (1995), p. 85.
17. S.A. David and T. DebRoy, MRS Bulletin, XIX
(1) (1994), p. 29.
18. T. Zacharia et al., Metall. Trans., 20A (1989),
p. 957.
19. K. Hong, D.C. Weckman, and A.B. Strong, Trends
in Welding Research, ed. H.B. Smartt, J.A. Johnson,
and S.A. David (Materials Park, OH: ASM Int., 1996),
p. 399.
20. R.T.C. Choo and J. Szekely, Weld J., 73 (1994),
p. 255.
21. W. Pitscheneder et al., Weld J., 75 (3) (1996),
p. 71s.
22. Y. Dong et al., Weld J., 76 (10) (1997), p. 442s.
23. J. Goldak et al., Mathematical Modeling of Weld
Phenomena 3, ed. H. Cerjak (London: Institute of
Materials, 1997), p. 543.
24. T. DebRoy, Proceedings of the Julian Szekely
Symposium on Materials Processing, ed. H.Y. Sohn,
J.W. Evans, and D. Apelian (Warrendale, PA: TMS,
1997), p. 365.
25. T. DebRoy et al., Mathematical Modeling of Weld
Phenomena 6, ed. H. Cerjak (London: Institute of
Materials, 2002), p. 21.
26. S.A. David and C.T. Liu, Weld J., 61 (1982), p. 157s.
27. M. Rappaz et al., Metall. Trans. 20A (1989), p. 1125.
28. S.A. David et al., Metall. Trans. A, 21A, (1990), p.
1753.
29. J.M. Vitek et al., Int’l. Trends in Welding Science and
Tech., ed. S.A. David and J.M. Vitek (Materials Park,
OH: ASM Int., 1993), p. 167.
30. J.M. Vitek et al., Sci. Technol. Weld. Joining, 6 (5)
(2001), p. 305.
31. B. Radhakrishnan and T. Zacharia, Modeling and
Control of Joining Processes, ed. T. Zacharia (Miami,
FL: Am. Weld. Soc., 1994), p. 298.
32. Ch.-A. Gandin, M. Rappaz, and R. Tintillier, Metall.
Trans., 24A (1993), p. 467.
33. W.B. Dress, T. Zacharia, and B. Radhakrishnan,
Modeling and Control of Joining Processes, ed. T.
Zacharia (Miami, FL: Am. Weld. Soc., 1994), p. 321.
34. S.S. Babu et al., Mater. Sci. Technol., 11 (1995), p.
186.
35. M.C. Flemings, Solidification Processing (New York:
McGraw Hill, 1974).
36. W. Kurz and D.J. Fisher, Fundamentals of
Solidification (Aedermannsdorf, Switzerland: Trans-Tech. Publications, 1986).
37. R. Trivedi et al., J. Appl. Phys., 93 April (2003) p.
4,885.
38. J.A. Brooks and M.I. Baskes, Advances in Welding
Science and Technology, ed. S.A. David (Materials
Park, OH: ASM Int., 1986), p. 93.
39. E. Scheil, Z. Metall, 34 (1942), p 70.
40. T. Matsumiya et al., Nippon Steel Technical Report 57 (1993), p. 50.
41. W.F. Savage, C.D. Lundin, and A. Aronson, Weld J., 44 (1965), p. 175.
42. T. Ganaha, B.P. Pearce, and H.W. Kerr, Metall.
Trans, 11A (1980), p. 1351.
43. H.W. Kerr and J.C. Villefuerta, Metal. Sci. of Joining, ed. K.J. Cieslak et al. (Warrendale, PA: TMS, 1991), p.
11.
44. J.M. Vitek et al., to be published in proceedings of
Thermec 2003, (Switzerland: Trans Tech Publishers)
Madrid, Spain.
45. S.A. David et al., Sci. Technol. Weld. Joining, 2 (2)
(1997), p. 79.
46. J.M. Vitek, S.A. David, and L.A. Boatner, Sci. Technol.
Weld. Joining, 2 (3) (1997), p. 109.
47. M. Gäumann, R. Trivedi, and W. Kurz; Mater. Sci.
Eng., A 226-228 (1997), p. 763.
48. T.M. Pollock and W.H. Murphy, Metall. Mater. Trans., 27A (1996), p. 1081.
49. J.M. Vitek and S.A. David, Laser Materials
Processing IV, ed. J. Mazumder, K. Mukherjee, and B.L.
Mordike (Warrendale, PA: TMS 1994), p. 153.
50. H.K.D.H. Bhadeshia, S.A. David, and J.M. Vitek,
Mater. Sci. Technol. 7 (1991) p. 50.
51. B. Sundman, B. Jansson, and J.O. Andersson,
Calphad, 9 (1985) p. 1.
52. J. Agren, ISIJ International, 32 (1992), p. 291.
53. S. Fukumoto and W. Kurz, ISIJ International, 38
(1998), p. 71.
54. U. Dilthey, V. Pavlik, and T. Reichel, Mathematical
Modeling of Weld Phenomena 3, ed. H. Cerjak (London:
Institute of Materials, 1997) p. 85.
55. A.C. Hall et al., Proceedings of the 11th International
Conference and Exhibition on Computer Technology in
Welding (Columbus, Ohio, 2001).
56. J.W. Elmer, J. Wong, and T. Ressler, Metall. Mater.
Trans., 29A (1998) p. 276.
57. S.S. Babu et al., J. Proc. Roy. Soc. A., 458 (2002),
p. 811.
58. S.S. Babu et al., Acta Materialia, 50 (2002), p. 4763.
59. J.W. Elmer, T. Palmer, and S.S. Babu, Adv. Mater.
Proc.,160 (2002) p. 23.
For more information, contact S.A. David, Oak Ridge National Laboratory, Metals & Ceramics Division, Building 4508, MS 6095, Oak Ridge, Tennessee 37831-6095; (865) 574-4804; fax (865) 574-4928; e-mail Davidsa1@ornl.gov.
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|