
An Article from the July 2002 JOM-e: A Web-Only Supplement to JOM
 |
TMS
ONLINE | TMS
PUBLICATIONS | SITE
MAP An Article from the July 2002 JOM-e: A Web-Only Supplement to JOM |
|
|
|
|
The
authors of this article are with King
Fahd University of Petroleum and Minerals,
Dhahran, Saudi Arabia.
|
Exploring traditional, innovative, and revolutionary issues in the minerals,
metals, and materials fields.
|
| OUR LATEST ISSUE |
|
|||
|
|
|||
|
FIGURES
|
|
|
1
|
 |
|
2
|
 |
|
3
|
 |
|
4
|
 |
|
5
|
 |
|
6
|
 |
|
7
|
 |
|
8
|
 |
|
9
|
 |
|
10
|
 |
|
11
|
 |
|
12
|
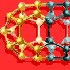 |
|
13
|
 |
|
14
|
 |
|
15
|
 |
|
16
|
 |
|
17
|
 |
|
18
|
 |
|
19
|
 |
|
20
|
 |
|
21
|
 |
|
22
|
 |
|
23
|
 |
|
24
|
 |
|
25
|
 |
|
26
|
 |
|
|
|
It is broadly accepted that materials science should be introduced using a
cross-disciplinary approach. Well-established, harmonized, and coordinated basic
disciplines—chemistry, physics, and mathematics—must be relied upon
and referred to. The terminology of materials science should comply with fundamental
disciplines. Moreover, further aspects of matter phenomena (e.g., thermal conductivity
or magnetic properties) should be introduced comprehensibly to students, in
advance of specialized courses such as thermal sciences, theory of plasticity,
or electronics. Looking at material forms and phenomena from diverse points
of view brings many benefits. The focus in the first year should be on academic
disciplines that categorize knowledge within formally defined domains. Materials
scientists should also learn from the resources of astrogeology. Many "artificial"
materials are only purified natural forms. Learning about conditions that have
caused the appearance of natural forms introduces valuable information into
materials engineering. The new gates for knowledge transfer are just opening
and we are still not fully aware of the possibilities made available by the
internet. Well-established worldwide strategy at engineering universities—to
build up such a formal educational base during initial semesters—enables
students to engage gradually in the realm of engineering materials during subsequent
years. Courses in applied (practical) engineering should be attended together
with a disciplined upgrade in advanced formal education (such as fluid mechanics,
thermodynamics, or electronics). From the point of view of materials science,
this schedule prepares students to face the global picture.
The possible global picture appearing out of such an exposure is inspiring
and exciting: eternal and infinite matter that assumes prodigious varieties
of form undergoing endless transmutations of motion. Novice students should
be presented with this fascinating and breathtaking vision; the students' ability
to look at this picture should not be underestimated.
Of course, the picture becomes much more attractive when it is demonstrated
that its magnification can be controlled by advancing technical means.
In 1981, G. Binnig and H. Rohrer of IBM
Research invented the scanning tunneling microscope. This device, easily
one of the most elegant and unanticipated inventions of the century, allowed
imaging of individual atoms and won Binnig and Rohrer the Nobel Prize in physics.
In 1985, Binnig and C. Gerber of IBM,
along with C. Quate of Stanford
University, invented the atomic force microscope. This allowed imaging nonconductive
matter such as living cells to molecular (although not currently atomic) resolution.
Since then, every year has seen new inventions in the rapidly growing field
of scanning probe microscopes, which are now imaging bits on magnetic surfaces,
measuring temperature at microscopic sites, and monitoring the progress of chemical
reactions (Figure
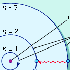
1).
Recently, scientists at IBM
in San Jose, California, discovered how to move atoms with a scanning tunneling
microscope and to position them in a pre-selected pattern (Figure
2).
Numerous images from the fractions of outer space we have reached thus far are
available to students on the internet.3
The same is true of images of fractions of inner space—engineering materials.
Students of materials science can review a variety of examples of magnified
microstructures on the internet (Figures 3
and 4).
In addition, the internet has brought in the wide spectrum of artificial intelligence
aids (software, programs, animations) that are available daily to students,
along with internal facilities provided by local networks as a part of learning
support.
The application of artificial intelligence aids and web-based resources have
become reality in materials education.6-8
Contemporary textbooks incorporate, as a rule, compact disk supplements including
a variety of artificial intelligence and multimedia aids.
The advantages of such interactive access to course material are numerous. Searching
for and linking information is less tedious and the combination of animations
with replay and capture are very educative and inspiring. The amount of knowledge
that can be accessed and communicated has been significantly shifted in a positive
direction. Of course, all traditional means continue to be available, and the
overall process of education still can be supervised by academics as appropriate.
A further option deserves to be highlighted. Artificial intelligence aids have
brought an additional capability into the classroom. The teacher can now afford
to be engaged with greater flexibility to satisfy student curiosity. When a
teacher responds promptly to questions (even when they somewhat digress from
the well-prepared course of the lecture) rather than suppressing them, the attention
of students is manifoldly increased. It should be admitted for once that the
teacher who is not equipped with artificial intelligence aids cannot possibly
respond to a barrage of questions of 20 or more really curious students.
This broad availability of artificial intelligence teaching and learning aids
has brought in a real opportunity to take advantage of curiosity-driven learning
(Figure
5).
A course in materials science should incorporate parallel construction of the
following concepts (along with presenting their historical and futuristic aspects):
selection and application of materials, development of processing techniques
(including manufacturing), and scientific comprehension of material forms.
It is clear that presenting complete varieties within each of the above topics
cannot be achieved during one educational course, even if it is a life-long
learning course. This leads to the question: How should the fragments of information
be selected? What makes some topics more educative, more instructional than
others? Apart from satisfying the condition of being related to the course (and
indeed, being true), the principal criteria for including certain information
in the course should include
Diverse examples of products-items from everyday life, as well as the usage
of technological achievements and the advantages of these accomplishments-should
be presented to students. Pictures of consumption items (from medicine, multimedia,
transportation, etc.) should be presented to students. Then a natural question
will appear: How can we produce and even improve this product? Questions will
initiate curiosity, which will trigger motivation for studying the nature of
matter forms and manufacturing technologies (Figure
5).
From the standpoint of application of the materials, inspiring examples should
be presented along with the notion of the material attributes. Material attributes
(properties) present the ultimate criteria of the suitability of the matter
form under consideration (Figures 6,
7,
8,
and 9).
From a multitude of applications, ceramics are selected as a suitable example.
It is certainly inspiring to learn that some types of ceramics, materials that
have seen a surge in popularity in recent decades, have been known for over
5,000 years. In the meantime, knowledge about ceramics has indeed advanced,
and nowadays engineers can produce nanoceramic structures such as shown in Figures
7,
8,
and 9.
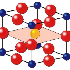
The structure shown in Figures 7,
8,
and 9,
called perovskite, usually has a cubic crystal lattice. However, in barium titanate
(BaTiO3), shown in Figure
9a, the central Ti4+ cation can be induced
to move off center, leading to a noncubic symmetry and to an electrostatic dipole,
or alignment of positive and negative charges toward opposite ends of the structure.
This dipole is responsible for the ferroelectric properties of barium titanate,
in which domains of neighboring dipoles line up in the same direction. The enormous
dielectric constants achievable with perovskite materials are the basis of many
ceramic capacitor devices.14
Further examples of advanced ceramics are refractories. Refractories make use
of the high melting points of ceramics, and are employed in great quantities
in the metallurgical, glassmaking, and ceramics industries, where they are formed
into a variety of shapes to line the interiors of furnaces and other devices
that process materials at high temperatures. Refractory ceramics have made inroads
as discrete components and as coatings for metallic components for combustion
engines. The outstanding wear and corrosion resistance combined with increased
toughness of zirconia ceramics present a prototype of design-for-purpose of
engineering materials (Figure
10).
Tribological properties of zirconia and zirconia/alumina composites at high
loads and high temperatures without lubricants are dynamically investigated.
For optimization of the microstructure, control is needed of the complete ceramic
fabrication procedure of the materials. For example, refractories made of zircon
(a zirconium silicate, ZrSiO4) are used in
glass tanks because of their good resistance to the corrosive action of molten
glasses.11,16,17
Further inspiring examples of demanding applications are engineering materials
used in space programs. There are, indeed, numerous other examples to be exploited
from the diverse domain of materials applications-perhaps certain variations
can be utilized over courses and semesters.
However broad, knowledge about material forms would be incomplete without understanding
processing and manufacturing techniques. We can utilize materials effectively
only if we are able to manufacture required products out of them. What use is
a material, however advanced, if a technique for bringing it into a desired
form cannot be mastered?
The evolution of mankind can be traced via the development of tools and techniques
over history.9
One important observation is the enormous acceleration of technological development
in recent centuries. The understanding of progressive aspects of technology
is of crucial importance. Over a long period of time, the history of manufacturing
highlights the moments of innovation that show this cumulative quality as some
societies advance to more sophisticated techniques. Manufacturing processes
are sources of large databases; they can be interpreted as gigantic statistical
experiments that provide a database for more complete understanding of material
forms.
Another important aspect is the transmission of technological innovations. The
modes of technology transmission have been enormously improved in recent centuries.
Trade in artifacts and technologies has ensured their widespread distribution
and encouraged imitation. The migration of craftsmen-whether the itinerant metalworkers
of early civilizations, the rocket engineers after World War II, or software
experts-has promoted the spread of new technologies for the space-age civilization.9,18
The importance of laboratory sessions and practical sessions where students
will experience physical contact with materials cannot be overemphasized. Yet,
clearly this laboratory practice has to be complemented with lectures where
actual exercise of imagination, abstract creativity, and capability of visualization
will take place. Only the combination of the above two aspects will enhance
the creativity in students of materials engineering.
The significance of the macroscopic world of our own dimensions, the scale of
real objects encountered in everyday life, should not be lost. At this very
instant our civilization figures within the limited space scale between the
planets and the atoms. Our knowledge, however, invades a much broader domain
that stretches between the universe and subatomic particles. We are already
using the matter forms from within that broader domain for magnetic fields,
plasma arc, scanning electrons, gravity, and various forms of radiations including
background cosmic radiation, within a variety of technologies.
As for now, we are capable of producing the temperatures of the stars, conductors
the sizes of synapses, and tubes the sizes of crystals (Figures 11
and 12).
Scientific understanding and knowledge should be presented along with such examples,
but care should be taken to avoid myths. A significant number of advances and
discoveries were the result of chance, fortunate outcomes of error, and, indeed,
dearly paid experience. Care should be taken not to inhibit students by pretending
that the scientists were supermen and the science is therefore hard to understand
or even beyond their reach. Scientific concepts are meant to be helpful in understanding
material forms. They provide both the intellectual satisfaction and the powerful
motor for curiosity-driven learning.
The brilliant models of the periodic table of chemical elements, the structure
of atoms, atom bonds, molecules, crystallography, phase diagrams, and numerous
other scientific theories are definitely concepts to be highlighted, relied
upon, and focused on within any course of materials science.
A history of our understanding of material forms should be given full attention
since it provides students with inspiring trends that can be used to anticipate
possible locations of answers they may be looking for. Knowledge can be structured
in layers and levels-in an analogy to the structure of matter itself—and
these layers should be connected by rationally highlighted key terms. A good
example of such a structure is the Encyclopaedia
Britannica Online. There are no limits to rearranging and restructuring
selected fragments of databases and expert systems to match the specific topic
and to follow the curiosity of the student.
It is highly educative to start lectures by showing how knowledge describing
the building blocks of matter makes progress over history. The early models
of the configuration of electrons and atomic nuclei resemble the arrangement
of planets orbiting the central stars such as our solar system.
Rutherford proposed in 1911 the model of an atom as a dense, positively charged
nucleus, in which nearly all the mass is concentrated, around which the light,
negatively charged electrons circulate at some distance (Figure
13). This model, also called the planetary model, was based wholly on classical
physics. It was superseded in a few years by the Bohr atomic model (Figure
14) incorporating some early quantum theory, and by the Shell model (Figure
15), all being today considered to be inferior compared to the collective
model, also called the unified model.
The concept of atoms consisting of a large portion of empty space where electrons,
protons, and neutrons occupy only minor volume has been replaced with concepts
of energy shells. The shell atomic model was proposed in 1949: Electrons are
thought of as occupying diffuse shells in the space surrounding a dense, positively
charged nucleus. Each shell accommodates only a specific number of electrons.
The shells extend outward and overlap one another. Different atoms have a different
numbers of electrons, which are distributed in a characteristic electronic structure
of filled and partially filled shells. The lightest element, hydrogen, has one
electron in the first shell only. The heaviest elements in their normal states
have only the first four shells fully occupied with electrons and the next three
shells partially occupied. All chemical elements are in some stage of constituting
or decay, as are the subatomic particles, the planets, and the stars.
The collective model describes the atomic nuclei by incorporating aspects of
the shell model and so-called "liquid-drop" model to explain certain
electromagnetic properties. In the shell model, nuclear behavior is explained
on the basis of unpaired nucleons (protons and neutrons) beyond the passive
nuclear core composed of closed shells of paired protons and paired neutrons.
In the liquid-drop model, nuclear behavior is explained via statistical contributions
of all the nucleons (much as the molecules of a spherical drop of water contribute
to the overall energy and surface tension). In the collective model, high-energy
states of the nucleus and certain magnetic and electric properties (moments)
are explained by the motion of the nucleons outside the closed shells combined
with the motion of the paired nucleons in the core. The nuclear core may be
thought of as a liquid drop on whose surface circulates a stable tidal bulge
directed toward the rotating unpaired nucleons outside the bulge. The tide of
protons (positively charged particles) constitutes a current that, in turn,
contributes to the magnetic properties of the nucleus, and the greater deformation
of the nucleus as the number of unpaired nucleons increases accounts for the
measured electric quadrupole moment (which may be considered an index of nuclear
shape, or a measure of how much the distribution of electric charge in space
departs from spherical symmetry).
Protons, which are considered to be a highly stable form of matter, are still
undergoing decay and re-generation processes (i.e., they fluctuate, or vibrate).
These oscillations fit well into the overall body of knowledge of eternal radiant
pervasive fluctuations.
The electron is the lightest 'stable' subatomic particle known. It carries a
negative charge that is considered the basic charge of electricity. An electron
has a resting mass of 9.1–10-28 gram,
which is only 0.0005 the mass of a proton. It has a half-integral spin. Spin
constitutes the property of intrinsic angular momentum in quantum-mechanical
terms. The electron reacts only by the electromagnetic, weak, and gravitational
forces; it does not respond to the short-range strong nuclear force that acts
between quarks and binds protons and neutrons in the atomic nucleus. The electron
has an antimatter counterpart called the positron, which has precisely the same
mass and spin but carries a positive charge. If it meets an electron, both are
annihilated in a burst of energy. Positrons are rare on the Earth, being produced
only in high-energy processes (e.g., by cosmic rays) and live only for brief
intervals before annihilation by electrons.
One graphic method of representing the interactions of elementary particles,
invented by R. Feynman, introduces diagrams as an aid to calculating the processes
that occur between electrons and photons, following the model of quantum electrodynamics.
In a Feynman diagram, now used to depict all types of particle interaction,
one axis represents space while the other represents time. Straight lines are
used to depict fermions-particles with half-integral values of intrinsic angular
momentum (spin), such as electrons (e-); and wavy lines are used for bosons—particles
with integral values of spin, such as photons.
At the quantum level, the interactions of fermions occur through the emission
and absorption of the field particles associated with the fundamental forces,
in particular the electromagnetic force, the strong force, and the weak force.
These field particles are all bosons. The basic interaction, therefore, appears
on a Feynman diagram as a vertex (i.e., a junction of three lines). In this
way, the path of an electron, for example, appears as two straight lines connected
to a third, wavy line where the electron emits or absorbs a photon (Figures
16
and 17).
An electron and its anti-matter counterpart, the positron, collide at high energy
(Figure
16). They are annihilated, and the energy is carried off as a photon, which
may then produce another pair of particles. The particles thus produced are
always a particle-antiparticle pair.
A macro-analogy for this sort of diagram can be imagined as a pair of ice dancers
skating toward one another. As they meet, one scoops the other up, and they
travel as one particle for while, then separate again.
Quantum mechanics predicts probabilities in matter (wave functions); however,
the mathematical calculations necessary to describe the probability states for
electrons in an atomic or molecular system were far too complex until W. Kohn
discovered in the 1960s that the total energy of an atomic or molecular system
described by quantum mechanics could be calculated if the spatial distribution
(density) of all electrons within that system were known. It was not necessary,
then, to describe the probable motions for each individual electron within such
a system, but merely to know the average electron density located at each point
within a system (Figure
18). Kohn's approach, the density-functional theory, greatly simplified
the computations needed to understand the electron bonding between atoms within
molecules. The method's simplicity enables researchers to map the geometrical
structure of even very large molecules and to predict complex enzymatic and
other chemical reactions.26
The contemporary development of increasingly powerful computers opened up new
opportunities: In the 1960s J. Pople designed a computer program, Gaussian,
that could perform quantum-mechanical calculations to provide theoretical estimates
of the properties of molecules and of their behavior in chemical reactions.
Gaussian is used in chemical laboratories throughout the world and has
become a basic tool in quantum-chemical studies. The computer models provided
by this program have increased the understanding of such varied phenomena as
interstellar matter and the effect of pollutants on the environment.27
Contemporary quantum chemistry describes properties of molecules in terms of
layers of electron densities. The interatomic surfaces are defined in terms
of a particular topological property of the electron density.28
The appearance of atoms is a consequence of the manner in which the electrons
are distributed throughout space in the attractive field exerted by the nuclei.
The nuclei act as point attractors immersed in a cloud of negative charge, the
electron density. The electron density describes the manner in which the electronic
charge is distributed throughout real space. The electron density, which is
a measurable property, determines the appearance and form of matter.29
This is illustrated in Figures 19,
20,
and 21.
The atoms are linked in molecules by the electron density, the glue of chemistry.
This approach provides a basis for a new pictorial approach to molecular structure.
Whereas most of the properties of simple molecules can be satisfactorily explained
in a non-relativistic quantum mechanical model, the properties of more complex
modules require the introduction of stochastic and relativistic models.32
Figure
21 shows the electron densities resulting from relativistic calculation
on UF6. The molecular orbital varies from
strongly anti-bonding to almost non-bonding due to the contraction of the U
6s orbital. Purple represents very low density and red is high density.31
The concepts presented above may be shown to students to build up their confidence
that a growing stock of knowledge is becoming available for their use. However,
care should be taken to avoid confusing students by making this excursion into
advanced regions of knowledge. It should be clarified that materials engineering
is a multidisciplinary subject and that the real-time performances are based
on the collaboration of teams of experts. The expertise is achieved by studying.
It should be demonstrated how the most complex concepts can be communicated
and presented in a clear way, by highlighting the relevant aspects in an appropriate
and simple manner. Although our knowledge has grown to gigantic proportions,
we can visit chosen regions and connect the points at the speed of electrons.
The structure of materials can be simplified to embrace the concepts of crystals,
microstructure phases, and other concepts that are important in applied materials
engineering.33
Figure
22 depicts motionless spheres ("hard sphere" model of atoms) while
Figure
23 shows actual atoms of germanium that are lined along the crystal plane
(111).
Figure
23 shows an atom as an object—a sphere. The notion of an object (entity)
as opposed to a process is a consequence of relativity of motion. "Process"
implies kinetics, a dominant change of relations, while "object" implies
the presence of a fixed structure and dominancy of constant attributes. In reality,
any arbitrary object continuously fluctuates its attributes due to its global
motion, due to motion of its structural constituents, and due to interfacial
motion.
Figure
23 is clear and simple to understand because the sample was cooled to extremely
low temperatures, nearly 200°C below ambient temperatures at which we would
normally encounter solid germanium. The temperature of the observed solid sample
was decreased in order to reduce atom vibrations and to enable the scanning
tunneling microscopy observations. At those low temperatures, the amplitude
of vibrations decreases to such an extent that we can distinguish individual
locations of atoms along the crystal plane.
Records of real material forms should be presented in a constructive (educative)
way that is adapted to our biological sensory reception, and, for that matter,
to our intellectual capabilities. Although natural interaction should not be
suppressed, the confusing effect of natural phenomena on our senses should be
obviously avoided. Images of matter phenomena in their natural form should be
processed not at the expense of the truth but for the purpose of clarification.
Figures 8
and 9
illustrate one example of such a clarification. Students can understand the
crystal structure of perovskite (Figure
8) only if it is drawn in a schematic mode shown in Figure
9. In addition, the application and the functionality of the crystal structure
presented in Figure
8 can be much better understood when presented as a schematic shown in Figure
24.
At the same time, students should be exposed to the variety of views of matter
at differing magnifications (Figures 25
and 26).
The sources of inspiration are diverse and the doors should not be closed to
the intrinsic attractiveness of material forms that can motivate students and
invoke their curiosity. Looking at the natural appearance of material forms
initiates growth of our intellectual capabilities. We have developed our primary
biological receptor senses to meet the challenges of our planet. We have developed
our intellectual capabilities to meet the challenges of the solar system. Can
we develop some new abilities (sensory receptors) to meet the challenges of
the micro-cosmos and the galaxies?
The journey into the world of material forms can be continued at higher and
higher magnifications. Advances in materials engineering occur on a daily basis.
Students should be told why the particular framework is drawn in a specific
way and the scope of the existing stock of knowledge underneath the surface
presented to them.
With the advent of artificial intelligence aids, the accessibility of information
and the connectivity of knowledge domains have risen to levels unheard of only
a dozen years ago. In today's artificial-intelligence equipped classroom, students
can access a worldwide network of information, they can perform multiple statistical
analyses of large databases, and communicate on-line with teams located at a
distant location. The development of artificial intelligence aids for the selection
of engineering materials has radically enhanced this task, which requires a
combination of expertise along with a workable overview of the existing and
potentially available engineering materials.38
Materials science should be presented to students keeping in mind the purpose
for which this knowledge is to be used. Certainly, some of the future purposes
can be anticipated and planning should be done accordingly to ensure that these
core needs are satisfied. Indeed, if a vision of future application of the introduced
knowledge is presented to students, their motivation for studying will increase.
Therefore, a portion of the knowledge should be presented in such a way as to
define relations that will be needed in performing planned engineering tasks.
Once these tasks are defined, a framework of the course should be designed to
enable students to access the corresponding knowledge. The limits—the scope
of knowledge to be presented—will have to be defined to enable evaluation
of the success in education.
Having satisfied this goal, an additional portion of knowledge should be presented
with no such restrictions except where the existing knowledge is limited by
the current state of the art. Of course, there will be natural restrictions
due to time limitations.
This brings in one fundamental question that deserves to be discussed in this
context, namely: What is the definition of the term "definition"?
A brief treatise on this topic is presented in the sidebar.
It is disturbing and confusing for students when topics in materials science
are introduced with statements such as "this problem is very complex".
Should that imply that phenomena of other disciplines are not so complex? Such
an approach is a consequence of unawareness of the actual dimensions of the
universe, its eternity and infinity. In reality, every phenomenon may be searched
infinitesimally deeper and infinitely broader providing that exhaustive explanations
of all aspects are sought. In connection with this, it is surprising that attention,
especially in education, is not devoted to explaining where the current boundaries
of our knowledge lie. By highlighting the boundaries of the known, and the adjacent
regions of unknown, positive intellectual potentials are stimulated to action
and inspiring questions are provoked.
If poetry is making beauty out of words, if painting is making beauty out of
shapes and colors, and if music is making beauty out of sounds, then science
and engineering are making beauty out of the universe.
|
DEFINING THE DEFINITIONS |
|
Definition A "DEFINITION" is a mirror image (a pattern, a model, an imitation, a reproduction), of some relation(s) that enables realization of a pre-selected change of some relation to be achieved by an entity that is capable of utilizing this definition for such a specified purpose. A definition cannot be generated (invented) without an entity, a system (material form), which exists at certain level of higher order and implies that the chaos within its domain is suppressed to a certain minimum degree. An example of such an entity is a human being. Another examples are some advanced levels of artificial intelligence systems. However, once it is generated, a definition can continue to exist (to be recorded) without the existence of the mentioned entity. A definition should be complemented with a minimum purpose statement (i.e., an explanation about a minimum domain of purposes for which the presented definition can be used). This statement does not exclude the possibility of using the definition correctly for some other purposes, it only specifies at least some minimum domain where the definition is applicable. It is useful if a definition is accompanied with highlighting the set of axioms that delimit the initial assumptions. If two definitions mutually contradict, one of them should be eliminated from the class of definitions. Such a disqualified information or contemplation should be included in the class of assumptions or hypotheses, or, if the probability of its erroneousness is high, it should be classified as a misconception (fallacy). For example, erroneous information or a mistaken theory do not qualify to be identified as definitions. For undecided information, terms "measurement", "notion", "signal", etc. can be used with a statement as to whether they are proven to be true and with what confidence. A hypothesis has not qualified to be a theory until it was proven with specified probability. For example, in information technology, the bytes are recording elements that become information only when they provide definition. The scientific language should attribute to each definition a unique set of words.
|
|
|
|
1. IBM-Scanning Tunneling Microscope Gallery; http://www.almaden.ibm.com/vis/stm/hexagone.html
(accessed 21 April 2001).
2. D.M. Eigler and E.K. Schweizer, "Positioning Single
Atoms with a Scanning Tunneling Microscope," Nature, 344 (1990), pp. 524-526;
IBM-Scanning Tunneling Microscope Gallery; http://www.almaden.ibm.com/vis/stm/atomo.html
(accessed 22 April 2001).
3. "The Hubble Deep Field," Space Telescope Science
Institute, NASA; http://oposite.stsci.edu/pubinfo/PR/96/01.html
(accessed 25 April 2001).
4. F. Muecklich and G. Petzov (expert committee) "Materialography,
MatFU e.K.; http://www.materialography.de/index_g.html
(accessed 25 April 2001).
5. F. Muecklich and G. Petzov (expert committee) "Materialography,"
MatFU e.K.; http://www.materialography.de/index_e.html
(accessed 24 April 2001).
6. D.J.M. Clark, "Developing,
Integrating, and Sharing Web-Based Resources for Materials Education,"
JOM-e,
50 (5) (1998).
7. C.J. McMahon, Jr., R. Weaver, and S.S. Woods, "Multimedia
Tutorials for an Introductory Course on the Science of Engineering Materials,"
JOM-e,
50 (5) (1998); (accessed 15 May, 2001).
8. V.R. Voller, S.J. Hoover, and J.F. Watson, "The
Use of Multimedia in Developing Undergraduate Engineering Courses,"
JOM-e,
50 (5) (1998); (accessed 15 May 2001).
9. S. Spuzic and J. O'Brien, "Fundamentals of Manufacturing"
(Artificial intelligence media files, excerpts are available on http://www.geocities.com/belaliki/welcome.html).
10. Encyclopædia
Britannica, Inc. 1994-2000, http://members.eb.com/bol/topic?tmap_id=7966000&tmap_typ=ip
(restricted access) (accessed 1 August 2000).
11. Inorganic Materials Science, Faculty of Chemical Technology;
http://ims.ct.utwente.nl/
(accessed 24 April 2001).
12. A. ten Elshof, "Dense Inorganic Membranes-Studies
on Transport Properties, Defect Structure and Catalytic Behaviour" (Ph.D.
Thesis,
University of Twente; http://ims.ct.utwente.nl/personen/docenten/tenelshof.html;
http://www.mesaplus.utwente.nl/masif/
(accessed 24 April 2001).
13. E. Span et al., "Pulsed Laser Ablation of LaSrCoO,"
Applied Surface Science, 150 (1999), pp. 171-177.
14. "Ceramic composition and properties," Encyclopædia
Britannica Online; http://members.eb.com/bol/topic?eu=114695&sctn=3
(restricted access) (accessed 2 May 2001).
15. J. E. ten Elshof, "Processing and Properties";
http://ims.ct.utwente.nl/algemeen/properties.html
(accessed 20 June 2002).
16. M. Matsuzawa, N. Yajima, and S. Horibe, "Damage
Accumulation Caused by Cyclic Indentation in Zirconia Ceramics," J. Materials
Sci.,
34 (1999), pp. 5199-5204.
17. "Refractory," Encyclopædia
Britannica Online; http://members.eb.com/bol/topic?eu=115219&sctn=1
(restricted access) (accessed 2 May 2001).
18. "Technology, history of," Encyclopædia
Britannica Online; http://www.eb.com:180/bol/topic?artcl=108659&seq_nbr=1&page=n&isctn=2
(restricted access) (accessed 31 May 2000).
19. "Carbon Tubes Reach Their Limit," BBC Science
News, 6 (November 2000), http://news.bbc.co.uk/hi/english/sci/tech/newsid_1009000/1009922.stm
(accessed 2 May 2001).
20. http://members.eb.com/bol/topic?asmbly_id=30896,
Encyclopædia Britannica
(restricted access) (accessed 30 January 2001).
21. http://members.eb.com/bol/topic?asmbly_id=1888,
Encyclopædia Britannica
(restricted access) (accessed 5 March 2001).
22. http://members.eb.com/bol/topic?asmbly_id=30921,
Encyclopædia Britannica
(restricted access) (accessed 5 March 2001).
23. http://walet.phy.umist.ac.uk/P615/Notes/Notes.html
(accessed 20 June 2002).
24. Copyright©1994-2000 Encyclopædia
Britannica, http://members.eb.com/bol/topic?asmbly_id=5671
(restricted access) (accessed 5 March 2001).
25. D. Kleppner and W. P. Spencer, Massachusetts Institute
of Technology, Encyclopædia Britannica, Copyright©1994-2000 http://members.eb.com/bol/topic?asmbly_id=7379
(restricted access) (accessed 5 March 2001).
26. "Kohn, Walter," Encyclopædia
Britannica Online; http://members.eb.com/bol/topic?eu=129633&sctn=1
(restricted access) (accessed May 2 2001).
27. "Pople, John A.," Encyclopædia
Britannica Online; http://members.eb.com/bol/topic?eu=129634&sctn=1
(restricted access) (accessed May 2 2001).
28. R. F.W. Bader, "Theoretical Investigations of Molecular
Structure and Reactivity," http://www.chemistry.mcmaster.ca/faculty/bader/;
and http://www.chemistry.mcmaster.ca/faculty/bader/aim/
(accessed May 2, 2001).
29. R.F.W. Bader, "What is an Atom?"; http://www.chemistry.mcmaster.ca/bader/aim/aim_1.html
(accessed 2 May 2001).
30. R.F.W. Bader, "The Topological Atom is the Quantum
Atom"; http://www.chemistry.mcmaster.ca/faculty/bader/aim/aim_3.html
(accessed 2
May 2001).
31. W.A.de Jong, Relativistic Quantum Chemistry Applied;
University of Groningen (1999) http://theochem.chem.rug.nl/~broer/Molfdir/Example.html
(accessed 2 May 2001).
32. M. Pernpointner, L. Vissscher, W.A. de Jong, and R.
Broer, "Parallelization of Four-Component Calculations. I. Integral Generation,
SCF, and Four-Index Transformation in the DiracFock Package MOLFDIR,"
J. Computational Chemistry, 21 (13) (2000), pp. 1176-1186; http://theochem.chem.rug.nl/~broer/Molfdir/Background.html
(accessed 2 May 2001); (also on http://tc.chem.vu.nl/~pernpoin/mopa.pdf).
33. Granta Design-Materials information technology (IT)
provider; http://www.granta.co.uk/
(accessed 16 May 2001).
34. W.D. Callister, Jr., Materials Science and Engineering,
4th edition (New York: Wiley,
1997).
35. http://dol1.eng.sunysb.edu/students/yuke/ge3.gif
(accessed 23 March 2001), the Surface Structure Group in the Department of Materials
Science
and Engineering Stony Brook University (sponsored by the National Science Foundation).
36. F. Muecklich and G. Petzov (expert committee) "Materialography,"
MatFU e.K.; http://www.materialography.de/index_g.html
(accessed 2 May 2001).
37. Spaceborne Imaging Radar-C/X-Band Synthetic Aperture
Radar (SIR-C/X-SAR) is a joint U.S.-German-Italian Project linked to NASA's
Mission to Planet Earth program, "Central Sumatra, Indonesia"; http://www.jpl.nasa.gov/radar/sircxsar/sumat.html
(accessed 2 May 2001).
38. Cornell Center for Materials Research, "News Nuggets";
http://www.ccmr.cornell.edu/nuggets/;
and http://www.ccmr.cornell.edu/nuggets/Disalvo.html
(accessed 27 March 2001).
39. S. Spuzic, "An Initiative in Improving Knowledge
Transfer in Engineering Education," Forum Proceedings from the 2nd Asia-Pacific
Forum on Engineering and Technology Education, ed. Zenon Pudlowski (1999),
p. 41; also available on http://www.geocities.com/belaliki/welcome.html.
40. Encyclopædia
Britannica Online and Merriam-Webster
Dictionary Online (1994-1999).
41. American Heritage Dictionary of the English Language,
3rd edition (New York: Houghton
Mifflin Company, Electronic Version 1995).
42. The 1997 Grolier Multimedia Encyclopaedia (Version
9.01M; Grolier Interactive,
1997).
For more information, contact Sead Spuzic, King
Fahd University of Petroleum and Minerals, Mechanical Engineering Department,
P.O. Box 1763, Dhahran, Eastern Province 31261, Saudi Arabia; +966-3-860-2840;
fax +966-3-860-2949; e-mail seadhana@kfupm.edu.sa.
Direct questions about this or any other JOM page to jom@tms.org.
| Search | TMS Document Center | Subscriptions | Other Hypertext Articles | JOM | TMS OnLine |
|---|