
An Article from the February 2005 JOM: A Hypertext-Enhanced Article
 |
TMS
ONLINE | TMS
PUBLICATIONS | SITE
MAP An Article from the February 2005 JOM: A Hypertext-Enhanced Article |
|
|
|
|
Manuel Elices is professor of materials science and department head, Gustavo V. Guinea is a professor, José Pérez-Rigueiro is associate professor, and Gustavo R. Plaza is assistant professor in the Materials Science Department. All are at the Universidad Politécnica de Madrid. Professor Guinea is also the president of the Spanish Structural Integrity Society. |
Exploring traditional, innovative, and revolutionary issues in the minerals, metals, and materials fields.
|
| OUR LATEST ISSUE |
|
Finding Inspiration in Argiope Trifasciata Spider Silk FibersManuel Elices, José Pérez-Rigueiro, Gustavo R. Plaza, and Gustavo V. Guinea |
The outstanding mechanical properties of silk fibers from the spider Argiope trifasciata are reviewed in this article, particularly the tensile behavior under controlled humidity and temperature. Samples obtained by forced silking showed a remarkable reproducibility. A novel procedure, wet stretching, developed by the authors, promises to shed light on the spinning of artificial silk fibers.
Spider silk is an unusually strong, resilient, and elastic fiber protein that is only surpassed—in some of its properties—by synthetic high-performance fibers. Table I compares some selected properties of silk fibers with those of several synthetic fibers. Silk fibers are nearly as strong as the manmade materials, and have an unbeatable capacity for absorbing energy, also called resilience, which can be quantified by the area under the stress-strain curve measured in a tensile test. This ability to store energy, and the fact that most of the energy is dissipated as the fiber deforms, enable spiders to intercept and catch their prey, absorbing their kinetic energy. This property makes silk fibers attractive for many applications in which energy absorption is the design parameter. Another interesting feature of silk fibers is the way they are produced: spiders spin silk in an aqueous medium, at room temperature, and from common materials. In addition, silk is recyclable and biodegradable.
Well-documented studies of spider silk properties have been published by Kaplan et al.,1 Gosline et al.,2 Viney,3 and Vollrath,4 among others. The production of natural and synthetic silk has been recently reviewed by Hinman et al.,5 Vollrath and Knight,6 Lazaris et al.,7 and Jin and Kaplan.8
In spite of the reported advances, the links between the structure and the mechanical properties of these outstanding fibers still remain largely unsolved, and little is known about the spinning process and the apparent effortlessness with which the spider spins the silk fibers.
In a pioneering work in 1976,9 M. Denny examined the mechanical properties of Araneus sericatus silk fibers. Since then, many other spider silk fibers have been studied, but how the primary structure of silk proteins dictates the mechanical behavior is still unknown. This question is pressing in the artificial synthesis of fibers inspired in spider silks obtained by bioengineering techniques. Recently, researchers from Nexia Biotechnologies, in cooperation with the Materials Science Team of the U.S. Army, described the successful synthesis of fibers spun from a solution of proteins obtained by transferring fragments of the corresponding spider gene into suitable mammalian cells.7 The mechanical properties of these artificial fibers, even if remarkable, still differ from those of their natural counterpart. It has been found that the conditions under which the fibers are spun and processed (by drawing, for example) are critical, and their influence in the spinning process is still unclear.
This article presents recent advances in the characterization and properties of spider silk fiber—particularly concerning its mechanical behavior—that could help to clarify the relationship between microstructure and properties, and the complexities of spinning. The article is focused on the silk of Argiope trifasciata (A. trifasciata, Argiopidae), a Mediterranean orb-web weaving spider which the authors have been studying since 2000. Like many other spiders, A. trifasciatas produce different kinds of silk fibers for various functions such as web building, prey immobilization, or cocoon silk. Due to space constraints, this article will concentrate on the strongest of the fibers produced, those used as the dragline and for web frames and radii, which are spun by the major ampullate gland.
The spider A. trifasciata and its major ampullate silk (MAS) fiber are shown in Figure 1. The fiber appears to be composed of two parallel monofilaments of circular cross sections known as brins. The double filament structure is known as a bave.
Little information is available on the protein structure of A. trifasciata MAS, but results from other species suggest that the fiber is made up of a protein network crosslinked with nanocrystallites of β-sheet10 microstructure. This network is anchored by hydrogen bond interactions established between different protein residues.11,12 Although not too detailed, this description of the microstructure captures some important features of spider silk and serves as a guide to interpret many of the findings presented in this work.
The MAS fibers from A. trifasciata are classified into two groups according to the collection procedure: those spun during the building of the web (radii, frame, and mooring lines), as well as the safety line, will be labeled as naturally spun (NS), and those obtained from an immobilized spider by forced silking are labeled forcibly silked (FS) (see the sidebar for details).
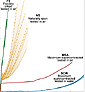
The tensile properties of NS fibers obtained directly from the web show a wide scatter of the stress-strain curves, as illustrated in Figure 2, which can be attributed in part to the intrinsic variability of biological materials.13 The flow of material through the spinning apparatus is under the active control of the spider,6 which suggests that the spider might be able to tailor the tensile behavior of the silk to match its intended use by varying the fiber diameter and microstructure. It was also observed that the stress-strain characteristics of a drag line produced in an undisturbed vertical climb are more reproducible than those of silk spun during horizontal crawling.14 From an experimental point of view, the variability in the properties of NS silk has been a major drawback since it has prevented the drawing of reliable conclusions.15
In contrast, FS fibers show acceptable reproducibility if a small number of precautions are taken,16 as is apparent in Figure 2. A low variability, and the possibility of collecting large quantities of silk in a simple, repetitive way have led to the adoption of forced silking as the preferred procedure for obtaining fibers for experiments.17 Most of the work in this paper is based on FS fibers. However, the tensile properties of FS fibers have been found to differ considerably from those of NS fibers: FS fibers are stiffer and show a smaller deformation at breaking than NS. Since both types have the same protein composition, the differences must be the result of changes in processing, as will be seen later.
FIGURES
|
|
1. |
 |
2. |
 |
3. |
 |
4. |
 |
5. |
 |
6. |
 |
7. |
 |
A. |
 |
FORCED SILKING |
Forced silking (FS) was first developed by Work & Emerson,17 and basically consists
of winding the silk fiber on a rotating cylinder. The spider must first be immobilized and
the silk thread stuck to the cylinder (Figure A). In the most elaborate configuration, the
cylinder follows an helicoidal movement, so that the fiber is placed in parallel rows on the
cylinder surface.17 This procedure allows spider silk in excess of five or six meters to be
retrieved in a few minutes. Also, the authors found that FS fibers from Argiope trifasciata
show a remarkable reproducibility.16 A number of studies attempting to determine the influence
of some parameters during FS (silking speed or anaesthetizing of the spider),15–28 are
under way. As FS shares some characteristics with the artificial spinning process, a better
knowledge of the influence of the silking parameters should be helpful in the design of
new artificial spinning techniques.
|
 |
Effect of Temperature and Moisture
To measure the small applied force on
the fibers, a 100 mN load cell with 0.1
mN resolution was used (HBM 1-Q11).
When needed, a precision balance Precisa
XT 220A with 0.001 mN resolution
was used to refine the results obtained
with the load cell. The fiber cross section,
required to scale forces into stresses,
was measured under a scanning-electron
microscope (JEOL 6300) and was
checked to ensure that the cross section
was almost circular.13 Any deformation
was either optically measured on the
fiber (Keyence LS-7500) or evaluated
from the relative displacement of the heads where the fiber tips were glued,
since the compliance of spider silk fibers
is at least 1,000 times larger than that of
the rest of the experimental setup.18 In
both cases, displacements were measured
within, at least, 1 µm resolution. The sensitivity of spider silk to the
environment, especially to moisture
content, required all the tests to be
performed under controlled conditions.
An environmental chamber (Dycometal
CCK-25/300) connected to a hydraulic
actuator (Instron A-1287) was the basic
experimental setup. A Rotronic I-2000
probe placed close to the fiber was
used for continuous measurement and
control of temperature and humidity.
Temperature and moisture were kept
within ±0.2°C and ±1% relative humidity
(RH), respectively. The influence of
the varying environmental conditions
on the experimental setup was carefully
checked. Particular attention was paid to
the performance of the extensometers
and the load cell, which were found to
operate correctly in the desired range of
experimental conditions. Prior to testing,
fibers were subjected to the prescribed
temperature and RH and allowed to stabilize
for at least one hour. A minimum
of three samples tested in each condition
showed, in general, very good reproducibility.
Details of the experimental
procedure are given elsewhere.18–20
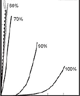
Figure 3 illustrates the influence of temperature and humidity on the stress strain curves of A. trifasciata FS fibers. Stress was obtained by dividing the force by the initial area of the fiber after conditioning. Strain was determined by dividing the change in length by the initial length of the fiber after conditioning.
Figures 3a, 3b, and 3c show stress strain curves at temperatures of 20ºC, 55ºC, and 90ºC and at different relative humidities. At any given temperature, the stiffness of the fiber decreases with increasing humidity. Conversely, when the moisture content is fixed, the stiffness of the fiber decreases with rising temperature, as illustrated in Figure 3d. For high enough values of humidity and temperature, the softening becomes extreme; the whole stress-strain curve changes its shape, resembling that of an elastomer.
Along with the overall slope of the stress-strain curve, the initial elastic modulus and the conventional yield stress decrease as temperature or RH (or both) increase, although the evolution of these parameters differs in the details. Tensile strength and strain at failure show less conclusive behavior, although a tendency to higher failure strain and lower tensile strength is seen with increasing humidity and/or temperature. The sensitivity of spider silk to environmental conditions can be accounted for from the network model mentioned in the introduction: increasing humidity and/or increasing temperature would disrupt some of the hydrogen bonds initially present in the dry silk, leading to more compliant fibers. At sufficiently high humidity and/or temperature, disruption of the hydrogen network would be almost complete and the tensile properties would be mainly dictated by the protein folding of the chains, leading to elastomeric behavior.
Effect of Time
The combination of a relatively labile
hydrogen bond network and the elastomeric
protein chain network also leads to
a noticeable evolution of the mechanical
properties of spider silk fiber with time,
reflected in two well-known phenomena
in the field of polymer science: physical
aging and viscoelasticity.
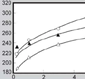
Physical aging is characteristic of polymers outside the equilibrium state and is related to transient disruption of the weak interactions and the rearrangement of the polymer chains. Figure 4a shows how aging affects silk fibers; it shows the evolution of the tensile stress-strain curves of FS fibers tested at different times after spinning. All the samples were stored and tested at a constant temperature of 20ºC and RH of 35%.
Aging leads to a slight increase of about 15% in the initial elastic modulus, as shown in Figure 4b. The conventional yield stress can show a significant increase of up to 30% from its initial value, as depicted in Figure 4c. In standard experimental conditions of 20ºC and 35% RH, the aging process appears to be completed within ten days, one month at most, after silking, since no noticeable evolution was observed after this time. However, the specific extent of the stabilization period may be largely dependent on the temperature and humidity of storage conditions, since physical aging is a thermally activated process also dependent on moisture content.21
The viscoelastic nature of silk fibers leads to time-dependent phenomena such as the relaxation of the tensile stress on the fiber when held under constant strain.
Stress-relaxation of FS fibers is illustrated in Figure 5a, in which only fully aged samples were used to obtain reliable and repetitive results, without the undesirable cross-effects of aging. Tests were performed at reference conditions of 20ºC and 35% RH. Stress relaxation in silk fibers depends largely on the initial stress level applied to the fiber. When the fiber is initially strained up to yield stress (upper curve in Figure 5a), the stress decay is close to 45% of the initial stress. This percentage falls close to 30% of the initial stress when the initial load level is halved. It is worth noting that stresses do not decay to zero, and they reach a constant value approximately within one day which, incidentally, is the typical spider-web life cycle.22
Viscoelastic effects are also present in tensile tests. Figure 5b shows the results of tensile tests on silk fibers performed over a wide range of strain rates, from 7 × 10–2 to 7 × 10–6 s–1. Stress-strain curves show that higher strain rates lead to stiffer responses and to an increment of all the relevant mechanical parameters: the initial modulus of elasticity increases by 14%, and yield stress, tensile strength, and breaking strain grow significantly. This behavior confirms the unparalleled dynamic performance of silk fibers, essential for intercepting flying prey. Both viscoelasticity and irreversible deformation account for the large hysteresis of more than 50% shown by silk fibers during stretch and recovery cycles and are responsible for the great energy dissipation of these fibers. Hysteresis is probably produced by frictional forces among segments of protein chains.23 Despite the excellent properties of spider silk, the picture that emerges from this discussion could seem discouraging if spider silk is to be considered as an engineering material: a future designer would have to deal with uncontrolled variability and sensitivity to the environmental conditions and the possibility that the properties of the material might change with time and could depend on the loading rate. Besides, the ability of spider silk to absorb and dissipate energy would lead to disposable devices, since these abilities depend on the irreversible deformation of silk. Fortunately, spider silk is endowed with some properties that make up for its major disadvantages and might compensate for the difficulties of designing with a material whose properties are time dependent. Interestingly, these properties are closely connected with supercontraction and the way the fiber is processed.
The mechanical properties of silk fibers are dependent not only on protein composition but also on the spinning process, which proceeds in an aqueous environment. This is why the study of silk fibers in aqueous media had attracted so much attention by researchers. The spinning of silk starts with a highly concentrated aqueous protein solution produced in a sac in the initial region of the silk glands.6 The sac is anchored by a funnel to an S-shaped duct that tapers down to the spigot. The initially isotropic protein solution acquires a liquid crystal structure in the funnel,8 so that it can flow more easily through the narrow duct. The liquid crystal structure could also promote the pre-alignment of the fibers prior to full fiber formation. The fiber is formed from the solution in the third limb of the duct, as seen from the separation of the lateral surface of the fiber from the walls of the duct,6 and emerges through the lips of the spigot. The identification of several structures along the duct and in the spigot responsible for recovering the water initially present in the aqueous solution suggests that water content might be a critical parameter during spinning.
In a water environment, MAS fibers undergo supercontraction.24 This effect is characterized by a large shrinkage of the fiber, exceeding 50% of its initial length, and by a dramatic change in its mechanical behavior. When tested in water, supercontracted fibers (the SCW fibers in Figure 2) show an elastomeric tensile behavior characterized by a very low initial elastic modulus and large (in excess of 100%) strain at breaking. When dried and tested in air, supercontracted fibers show an initial elastic regime with high stiffness, followed by a large plateau and a strain at breaking that exceeds 100%. This state is labeled as maximum supercontraction tested in air (MSA) (see Figure 2).25
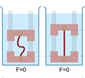
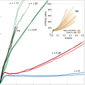
The search for a biological role of supercontraction, possibly in combination with stretching, led the authors to consider the processing of the fiber as a situation in which the fiber would be naturally subjected to both effects. In this context, the whole range of tensile properties displayed by naturally spun silk fibers have been mimicked through a combination of supercontraction and stretching. The results suggest that their combined effect plays a significant role during the spinning of spider silk. This study shows that the whole range of tensile properties exhibited by spider silk14 can be predictably reproduced by simply controlling the deformation of supercontracted fibers stretched in water, a process called wet stretching (Figure 6).26 In this procedure, an FS fiber glued by its ends to an aluminum foil frame is immersed in water at 20ºC. It is allowed to contract unrestrained up to the supercontracted length, stretched up to the selected length, and the ends are clamped in this position. Water is removed after 10 min., and the FS fiber is dried overnight at 20ºC and 35% RH. Finally, the stresses built up in the fiber during drying are relaxed by unloading down to the final length. Since the fiber is subjected to similar influences during spinning, the results indicate a critical biological function of supercontraction during the spinning process of silk fibers.
The effect of stretching on supercontracted fibers and subsequent drying is illustrated in Figure 7. The stretching process is characterized by the alignment parameter defined as α = LC/LSC-1 (see the inset in Figure 7 for definitions of LC and LSC). Very good repeatability—unusual when dealing with spider silk fibers—was achieved with this process, as is shown by the similar stress-strain curves presented by the three fibers tested for each alignment parameter.
A value of zero for the alignment parameter (α = 0) corresponds to dried supercontracted fibers (MSA). It was found that MSA fibers represent a lower limit of the tensile properties that can be reached by spider silk fibers tested in air, regardless of the previous loading history of the fiber.27
Stretching the supercontracted fiber leads to an increase in LC and consequently of the alignment parameter. The collection of stress-strain curves displayed by NS fibers (see inset in Figure 7) is regained for alignment parameters ranging from α ≈ 0.4 to α ≈ 0.8, and is illustrated by the curves corresponding to α ≈ 0.45. Alignment parameters in the range of α ≈ 0.9 to α ≈ 1.1 yield fibers with stress-strain curves similar to those of forcibly silked fibers (FS fibers are shown for direct comparison). These values of the alignment parameter indicate that the length of FS fibers doubles that of supercontracted fibers, as expected from the approximate 50% reduction of the length of FS fibers when subjected to supercontraction. From a microstructural point of view, the stretching and drying process on supercontracted fibers can be understood from the protein network model: the water molecules disrupt the hydrogen bond network, and further stretching rearranges the protein chain network to a new conformation. The final conformation is frozen by re-establishing a new hydrogen bond network after drying. This suggests that a similar stretching and drying process within the silk gland can account for the variability observed in the tensile properties of MAS fibers and places supercontraction as a central feature of the spinning process. Besides, the low forces involved in stretching supercontracted fibers up to large deformations (strain values up to 1.0) allow the modification of the tensile properties with a minimum expense of energy. From a technological perspective, the stretching and drying process allows the production of spider silk fibers with a tailored stress-strain profile in a reliable and repetitive way—even starting with irreversibly deformed fibers—which leads to the possibility of designing re-usable devices. This procedure casts light on the natural spinning process and can be helpful in designing the spinning processes that the biomimetics industry is developing.
This study shows the need for a better understanding of the structure-property relations in spider silk fibers, particularly:
It is worth noting that the final goal is not to copy the spider silk but to find inspiration in spider silks to design high performance fibers. Finally, one should remember that spiders remake their webs daily and Argiope, in particular, works in high moisture (RH = 60% and higher).
The authors thank K.K. Chawla for his kind invitation and encouragement to prepare this review paper. The authors also want to thank José Miguel Martínez for his help in setting up the experiments and the editing of the manuscript. A. trifasciata spiders were kindly provided by Jesús Miñano and reared by Oscar Campos and Iván Blanco (Naturaleza Misteriosa, Parque Zoológico de Madrid). This work was supported by Ministerio de Educación y Ciencia and Comunidad de Madrid, Spain, through grants MAT 2003-4906 and 07N/0001/2002.
1. D.L. Kaplan et al., “Silks,” Biomaterials. Novel
Materials from Biological Sources, ed. D. Byrom (New York: Stockton Press, 1991), pp. 1–53.
2. J.M. Gosline et al., “The Mechanical Design of
Spider Silks: From Fibroin Sequence to Mechanical
Function,” J. Exp. Biol., 202 (1999), pp. 3295–3303.
3. C. Viney, “Silk Fibres: Origins, Nature and
Consequences of Structure,” Structural Biological
Materials, ed. M. Elices (Amsterdam: Pergamon Press,
2000), pp. 293–333.
4. F. Vollrath, “Strength and Structure of Spiders’ Silks,”
Rev. Mol. Biotech., 74 (2000), pp. 67–83.
5. M.B. Hinman, J.A. Jones, and R.V. Lewis, “Synthetic
Spider Silk: A Modular Fiber,” TIBTECH, 18 (2000), pp.
374–379.
6. F. Vollrath and D.P. Knight, “Liquid Crystalline Spinning of Spider Silk,” Nature, 410 (2001), pp. 541–548.
7. A. Lazaris et al., “Spider Silk Fibers Spun from
Soluble Recombinant Silk Produced in Mammalian
Cells,” Science, 295 (2002), pp. 472–476.
8. H.-J. Jin and D.L. Kaplan, “Mechanisms of Silk
Processing in Insects and Spiders,” Nature, 424
(2003), pp. 1057–1061.
9. M.W. Denny, “The Physical Properties of Spider’s
Silk and Their Role in the Design of Orb-Webs,” J. Exp.
Biol., 65 (1976), pp. 483–506.
10. A.H. Simmons, C.A. Michal, and L.W. Jelinski, “Molecular Orientation and Two-Component Nature
of the Crystalline Fraction of Spider Dragline Silk,”
Science, 272 (1996), pp. 84–87.
11. J.M. Gosline, M. Denny, and M.E. DeMont, “Spider
Silk as Rubber,” Nature, 309 (1984), pp. 551–552.
12. Y. Termonia, “Molecular Modelling of the Stress/Strain Behavior of Spider Dragline,” Structural
Biological Materials, ed. M. Elices (Amsterdam:
Pergamon Press, 2000), pp. 337–349.
13. J. Pérez-Rigueiro et al., “Tensile Properties of
Argiope trifasciata Drag Line Silk Obtained from the
Spider’s Web,” J. Appl. Polym. Sci., 82 (2001), pp.
2245–2251.
14. M.A. Garrido et al., “Active Control of Spider Silk
Strength: Comparison of Drag Line Spun on Vertical
and Horizontal Surfaces,” Polymer, 43 (2002), pp.
1537–1540.
15. B. Madsen, Z.Z. Shao, and F. Vollrath, “Variability
in the Mechanical Properties of Spider Silks on Three
Levels: Interspecific, Intraspecific and Intraindividual,”
Int. J. Biol. Macromol., 24 (1999), pp. 301–306.
16. G.V. Guinea et al., “Reproducibility of the Tensile
Properties of Spider (Argiope trifasciata) Silk Obtained
by Forced Silking,” J. Exp. Zool., 303A (2004), pp.
37–44.
17. R.W. Work and P.D. Emerson, “An Apparatus
and Technique for the Forcible Silking of Spiders,” J.
Arachnol., 10 (1982), pp. 1–10.
18. J. Pérez-Rigueiro et al., “Silkworm Silk as an
Engineering Material,” J. Appl. Polym. Sci., 70 (1998), pp. 2439–2447.
19. J. Pérez-Rigueiro et al., “Mechanical Properties of
Silkworm Silk in Liquid Media,” Polymer, 41 (2000), pp.
8433–8439.
20. G.V. Guinea et al., “Self-tightening of Spider Silk
Fibers Induced by Moisture,” Polymer, 44 (2003), pp.
5785–5788.
21. G.R. Plaza, “Thermo-hygro-mechanical Behaviour
of Spider Silk Fibers,” [Ph.D. Thesis (in Spanish)
Universidad Politécnica de Madrid, 2004].
22. R.F. Foelix, Biology of Spiders (Oxford, U.K.: Oxford
University Press, 1996).
23. J.M. Gosline et al., “Elastomeric Network Models
for the Frame and Viscid Silks from the Orb Web of the
Spider Araneus diadematus,” Amer. Chem Soc. Symp.
Series 544 (1994), pp. 328–341.
24. R.W. Work, “Dimensions, Birefringences, and
Force-Elongation Behavior of Major and Minor
Ampullate Silk Fibers from Orb-Web-Spinning Spiders.
The Effects of Wetting on These Properties,” Textile
Res. J., 47 (1977), pp. 650–662.
25. J. Pérez-Rigueiro, M. Elices, and G.V. Guinea, “Supercontraction Tailors the Tensile Properties of
Spider Silk,” Polymer, 44 (2003), pp. 3733–3736.
26. G.V. Guinea et al., “Stretching of Supercontracted
Fibers: A Link between Spinning and the Variability of
Spider Silk,” J. Exp. Biol., 208 (2005), pp. 25–30.
27. M. Elices et al., “Recovery in Spider Silk Fibers,” J.
Appl. Pol. Sci., 92 (2004), pp. 3537–3541.
28. B. Madsen and F. Vollrath, “Mechanics and
Morphology of Silk Drawn from Anesthetized Spiders,”
Naturwissenschaften, 87, (2000) pp. 148–153.
For more information, contact Manuel Elices, Universidad Politécnica de Madrid, Departamento de Ciencia de Materiales, ETS de Ingenieros de Caminos, Ciudad Universitaria, 28040 Madrid, Spain; e-mail melices@mater.upm.es.
Direct questions about this or any other JOM page to jom@tms.org.
| SearchTMS Document CenterSubscriptionsOther Hypertext ArticlesJOM | TMS OnLine |
|---|