|
Lithium-ion battery technology is
projected to be the leapfrog technology
for the electrification of the drivetrain
and to provide stationary storage
solutions to enable the effective
use of renewable energy sources. The
technology is already in use for low power
applications such as consumer
electronics and power tools. Extensive
research and development has enhanced
the technology to a stage where
it seems very likely that safe and reliable
lithium-ion batteries will soon be
on board hybrid electric and electric
vehicles and connected to solar cells
and windmills. However, the safety of
the technology is still a concern, service
life is not yet sufficient, and costs
are too high. This paper summarizes
the state of the art of lithium-ion battery
technology for non-experts. It lists
materials and processing for batteries
and summarizes the costs associated
with them. This paper should foster an
overall understanding of materials and
processing and the need to overcome
the remaining barriers for a successful
market introduction.
INTRODUCTION
Worldwide battery demand mainly
driven by consumer electronics and
electric power tools is projected to rise
at a 6.9% annual rate through 2010 to
$73.6 billion.1
The effective use of low-emission
and emission-free energy sources, such
as renewable—but intermittent—wind
and solar energy, demands stationary, high-yield, long-lasting, and low maintenance
electrical energy storage
solutions. In 2006, Germany, the leading
nation in wind energy utilization as
a part of its overall energy production
portfolio, wasted 15% of its wind-produced
energy due to the lack of suitable
electrical energy storage.2
Hybrid electric vehicles (HEVs) and
all-electric vehicles (EVs) can reduce
the U.S. dependence on foreign oil and
will contribute to battery demand in the
future. Counting engine efficiencies
and including electrical energy production,
EVs could reduce the use of gasoline
to one-fourth of today’s consumption
and could reduce the U.S. dependence
on imported oil to one-sixth of
today’s level.3
The focus of the U.S. Department of
Energy’s (DOE’s) Vehicle Technologies
Program is on lithium-ion-based
electrochemical energy storage due to
the electrochemical potential and theoretical
capacity provided by that system.
Lithium-ion batteries can provide
a reliable rechargeable storage technology.
Developments in this program
include lithium-ion, lithium-ion-polymer,
and lithium-metal technology.
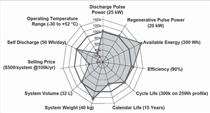
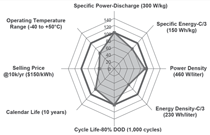
The DOE’s short-term goals for
power-assist HEVs are met or exceeded
in eight of 11 areas, showing the tremendous
success of the program. The
eight areas include discharge pulse
power, regenerative pulse power, available
energy, efficiency, cycle life, system
weight, system volume, and self
discharge. Still, three goals seem to be
more challenging and remain unmet:
operating temperature from –30°C
to 52°C, a lifetime of 15 years, and a
selling price below $500 to $800 per
system at 100,000 units produced per
year.4 For plug-in hybrid electric vehicles
(PHEVs) in the intermediate term and for EVs in the long term, accomplishments
are far from meeting the
goals, and significant material and processing
technological barriers need to
be overcome. Figure 1 illustrates the
DOE and U.S. Advanced Battery Consortium
(USABC) goals and milestones
met for HEV and EV applications.
The DOE program is focused on
overcoming the technical barriers associated
with HEV battery technology,
namely cost, performance, safety, and
life:6
- Cost—Current lithium-ion-based
battery cost per kilowatt is approximately
a factor of 2 too high.
The main costs are associated with
the high cost of raw materials and
materials processing as well as the
costs of the cell, packaging, and
manufacturing.
- Performance—Performance barriers
are mostly related to reduced
discharge power at low temperature
and loss of power due to use
and aging.
- Safety—Actual lithium-ion battery
technology is not intrinsically
safe. Short circuit, overcharge,
over-discharge, crush, and high
temperature can lead to thermal
runaway, fire, and explosion.
- Life—Hybrid engine systems
have an estimated 15 year lifetime.
Battery technology needs to meet
this target with a goal of 300,000
charging cycles. The cycle life has
been demonstrated but the calendar
life has not.
Historically, electrochemistry and
device engineering have dominated the
development of batteries. The above mentioned
performance barriers are
materials-related problems. Poor low temperature
performance is a diffusion
problem at low temperature. Loss of
power due to use is mostly a problem
related to mechanical behavior, crack
initiation and growth followed by fatal
fracture, and subsequent coating and
passivation of surfaces. Additionally,
materials development and materials-processing
development need to be
addressed in concert in order to reduce
cost and create a safe battery technology.
Therefore, materials scientists and
process engineers are slowly entering
the arena in which the goal of reliable,
safe, and long-lasting electrical energy
storage will be achieved.
BATTERY PRINCIPLE AND BASICS
|
HOW WOULD YOU...
|
…describe the overall significance
of this paper?
Lithium-ion battery technology
needs to overcome significant
technological, safety, and cost
barriers to be successful in
the marketplace. Traditionally,
battery technology was driven
by electrochemical R&D. Today,
materials scientists and process
engineers can help in overcoming
the barriers and understanding
failure mechanisms. This paper
educates materials scientists and
engineers to start that process.
…describe this work to a materials
science and engineering professional
with no experience in your
technical specialty?
Lithium-ion battery technology
is projected to be the leapfrog
technology for the electrification
of the drivetrain and to provide
stationary storage solutions
to enable the effective use of
renewable energy sources.
However, safety of the technology is
still a concern, service life is not yet
sufficient, and costs are too high.
This paper summarizes the state
of the art of lithium-ion battery
technology for nonexperts and
fosters understanding for materials
scientists and process engineers.
…describe this work to a layperson?
Hybrid and all-electric vehicles
and renewable wind and solar
power rely on efficient energy
storage. However, available
battery technology needs to
overcome significant barriers
in cost and efficiency to become
reliable and safe enough to work
as mobile or stationary storage.
Materials scientists and engineers
are working to increase their
reliability and reduce their cost
to become a safe and affordable
solution for our energy crisis.
|
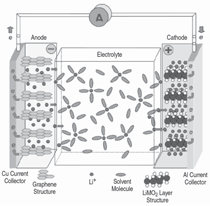
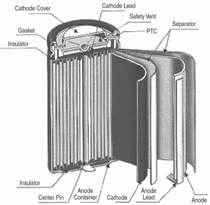
The smallest working unit in a battery
is the electrochemical cell, consisting
of a cathode and an anode separated
and connected by an electrolyte. The
electrolyte conducts ions but is an insulator
to electrons. In a charged state,
the anode contains a high concentration
of intercalated lithium while the
cathode is depleted of lithium. During
the discharge, a lithium ion leaves the
anode and migrates through the electrolyte
to the cathode while its associated
electron is collected by the current
collector to be used to power an electric
device (illustrated in Figure 2).
The cell designs and combinations
in modules and packs differ greatly.
To establish a base understanding, this
paper shows the main cell designs and
then focuses on materials, processing,
and manufacturing with special emphasis
on batteries for transportation.
The electrodes in lithium-ion cells
are always solid materials. One can
distinguish between cell types according
to their electrolytes, which may be
liquid, gel, or solid-state components.
The electrolytes in gel and solid-state
cells represent a structural component
and do not need additional separators
for the effective separation of electrodes
and avoidance of short circuits.
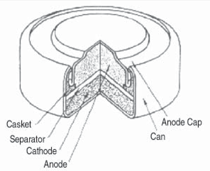
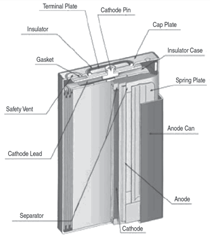
Cells come in button, cylindrical, and
prismatic forms (see Figure 3). A good
overview of the cell forms and materials
is provided by J. Besenhard et al.9
For low-energy and low-power applications,
a cell often represents a
full battery. For high-energy and high-power
applications such as transportation
or stationary storage, a number of
cells are packaged in a module, and a
number of modules are packaged in a
battery.
Thin-Film Batteries
A special category is the solid-state
thin-film battery. Thin-film batteries
consist of only solid materials. The
electrolyte is a solid-state ionic glass or
crystal, and the components are deposited
via vapor deposition techniques.
This design offers the highest energy
density, safety, and abuse tolerance, but
it is only applicable to small devices
for special applications and involves
the most costly production method. A
good review on thin-film battery systems
is provided by N.J. Dudney and
B.J. Neudecker.10
MATERIALS
Cathode Materials
State-of-the-art cathode materials
include lithium-metal oxides
[such as LiCoO2, LiMn2O4, and
Li(NixMnyCoz)O2], vanadium oxides,
olivines (such as LiFePO4), and rechargeable
lithium oxides.11,12 Layered
oxides containing cobalt and nickel are
the most studied materials for lithium-ion
batteries. They show a high stability
in the high-voltage range but cobalt
has limited availability in nature and is
toxic, which is a tremendous drawback
for mass manufacturing. Manganese
offers a low-cost substitution with a
high thermal threshold and excellent
rate capabilities but limited cycling behavior.
Therefore, mixtures of cobalt,
nickel, and manganese are often used
to combine the best properties and minimize
the drawbacks. Vanadium oxides
have a large capacity and excellent kinetics.
However, due to lithium insertion
and extraction, the material tends
to become amorphous, which limits the
cycling behavior. Olivines are nontoxic
and have a moderate capacity with low
fade due to cycling, but their conductivity
is low. Methods of coating the material
have been introduced that make
up for the poor conductivity, but it adds
some processing costs to the battery.
Anode Materials
Anode materials are lithium, graphite,
lithium-alloying materials, intermetallics,
or silicon.11 Lithium seems to be
the most straight forward material but
shows problems with cycling behavior
and dendritic growth, which creates
short circuits. Carbonaceous anodes are
the most utilized anodic material due to
their low cost and availability. However,
the theoretical capacity (372 mAh/g)
is poor compared with the charge
density of lithium (3,862 mAh/g).
Some efforts with novel graphite varieties
and carbon nanotubes have
tried to increase the capacity but have
come with the price of high processing
costs. Alloy anodes and intermetallic
compounds have high capacities but
also show a dramatic volume change,
resulting in poor cycling behavior. Efforts
have been made to overcome the
volume change by using nanocrystalline
materials and by having the alloy
phase (with Al, Bi, Mg, Sb, Sn, Zn,
and others) in a nonalloying stabilization
matrix (with Co, Cu, Fe, or Ni).
Silicon has an extremely high capacity
of 4,199 mAh/g, corresponding with a
composition of Si5Li22. However, cycling
behavior is poor, and capacity
fading not yet understood.
Electrolytes
A safe and long-lasting battery needs
a robust electrolyte that can withstand
existing voltage and high temperatures
and that has a long shelf life while offering
a high mobility for lithium ions.
Types include liquid, polymer, and
solid-state electrolytes.11 Liquid electrolytes
are mostly organic, solventbased
electrolytes containing LiBC4O8
(LiBOB), LiPF6, Li[PF3(C2F5)3], or
similar. The most important consideration
is their flammability; the bestperforming
solvents have low boiling
points and have flash points around
30°C. Therefore, venting or explosion
of the cell and subsequently the battery
pose a danger. Electrolyte decomposition
and highly exothermic side reactions
in lithium-ion batteries can create
an effect known as “thermal runaway.”
Thus, selection of an electrolyte often
involves a tradeoff between flammability
and electrochemical performance.
Separators have built-in thermal shutdown
mechanisms, and additional external
sophisticated thermal management
systems are added to the modules
and battery packs. Ionic liquids are under
consideration due to their thermal
stability but have major drawbacks,
such as lithium dissolution out of the
anode.
Polymer electrolytes are ionically
conductive polymers. They are often
mixed in composites with ceramic
nanoparticles, resulting in higher conductivities
and resistance to higher
voltages. In addition, due to their high
viscosity and quasi-solid behavior,
polymer electrolytes could inhibit lithium
dendrites from growing13 and could
therefore be used with lithium metal
anodes.
Solid electrolytes are lithium-ion
conductive crystals and ceramic glasses.
They show a very poor low-temperature
performance because the lithium
mobility in the solid is greatly reduced
at low temperatures. In addition, solid
electrolytes need special deposition
conditions and temperature treatments
to obtain acceptable behavior, making
them extremely expensive in use,
although they eliminate the need for
separators and the risk of thermal runaway.
Separators
A good review of separator materials
and needs is provided by P. Arora
and Z. Zhang.14 As its name suggests,
the battery separator separates the two
electrodes physically from each other,
thus avoiding a short circuit. In the case
of a liquid electrolyte, the separator is
a foam material that is soaked with
the electrolyte and holds it in place.
It needs to be an electronic insulator
while having minimal electrolyte resistance,
maximum mechanical stability,
and chemical resistance to degradation
in the highly electrochemically active
environment. In addition, the separator
often has a safety feature, called “thermal
shutdown;” at elevated temperatures,
it melts or closes its pores to shut
down the lithium-ion transport without
losing its mechanical stability. Separators
are either synthesized in sheets
and assembled with the electrodes or
deposited onto one electrode in situ.
Costwise, the latter is the preferable method but poses some other synthesis,
handling, and mechanical problems.
Solid-state electrolytes and some polymer
electrolytes need no separator.
PROCESSING AND MANUFACTURING
Battery discharge is based on the diffusion
of lithium ions from the anode
to the cathode through the current collector,
as shown in Figure 2. This moving
mechanism is primarily based on
diffusion processes: delivering lithium
ions to the surface of the anode, transitioning
to and diffusion through the
electrolyte, and transitioning to and
diffusion into the cathode. Diffusion is
the most limiting factor in high-current
discharge and charge as well as in low temperature
performance. In addition,
the intercalation and deintercalation
processes create a volume change in the
active electrode materials. This repeated
process due to cycling can initiate
cracks and can lead to eventual fracture
with the result of unusable active electrode
material due to disconnection to
the current collector or a short circuit
and—in case of lithium-metal batteries—a safety hazard due to roughening
of the anode and dendritic growth.
Efforts in materials processing and
manufacturing to increase performance
and to manage unavoidable volume
change have been leading toward composite
materials with micro- and nanoscaled
particles. Nanoparticles can accommodate
volume change with minimal
risk of crack initiation, and their
micro-scaled agglomerates and composites
result in minimal diffusion
path lengths through the slow diffusion
phases (electrodes). A strong focus is
on packing density to maximize active
material content, open porosity to access
the electrolyte, and electronic continuity
to guarantee charge exchange to
the current collectors.
Cylindrical cells are manufactured
and assembled as follows. The electrolytes
are formed from pastes of active
material powders, binders, solvents,
and additives and are fed to coating
machines to be spread on current collector
foils, such as aluminum for the
cathode side and copper for the anode
side. Subsequent calendaring for
homogeneous thickness and particle
size is followed by slitting to the correct
width. The components are then
stacked to separator-anode-separator cathode
stacks followed by winding to
cylindrical cells, insertion in cylindrical
cases, and welding of a conducting
tab. The cells are then filled with
electrolyte. The electrolyte has to wet
the separator, soak in, and wet the electrodes.
The wetting and soaking process
is the slowest step and therefore
is the determining factor in the speed of the line. All other needed insulators,
seals, and safety devices are then attached
and connected. Then, the cells
are charged the first time and tested.
Often cells have to be vented during the
first charge. First charging cycles follow
sophisticated protocols to enhance
the performance, cycling behavior, and
service life of the cells. Recently, efforts
have been made in combined and
hybrid processing, such as direct deposition
of separators onto electrodes and
rapid heat treatments.
COST ANALYSIS FOR BATTERIES FOR TRANSPORATION
The battery pack requirements for
HEVs are different from those for
PHEVs and EVs.6 The DOE’s program
production price targets are $500 to
$800 for HEV battery packs and $1,700
to $3,400 for the PHEV battery packs.
Material Needs and Raw
Material Cost
The raw material needs and costs are
based on a study by L. Gaines and R.
Cuenza.15 A standard cylindrical cell
is the so-called “18650 cell” (18 mm
wide and 65 mm long) which has a total
mass of about 40 g (including inactive
material and packaging) and a capacity
of about 1.35 Ah.16 The masses of material
needed for HEV and EV battery
cells are shown in Table I.
From Table I, one can estimate that
a cell’s capacity roughly scales with its
mass. Although the packaging as a part
of the whole for a large battery is smaller
than for a small battery, the total mass
of a 10 Ah cell is roughly 325 g and the
total mass of a 100 Ah cell is roughly
3,430 g. Thus, the cost calculations for
materials can be made by scaling up the
costs of materials in an 18650 cell by a
factor of 10 for HEVs and by a factor
of 100 for EVs. Most battery designs
result in batteries with a total of about
100 cells in a number of modules (such
as 12 × 8, 10 × 10, or similar).
Table I. Estimated Materials Content of Typical Lithium-Ion Cells (based on Reference 15)
|
| |
High-Energy (100 Ah) Cell EV
|
High-Power (10 Ah) Cell HEV
|
| Material/Component |
Quantity
(g)
|
Part (%)
|
Quantity (g)
|
Part
(%)
|
| Anode (dry) |
|
|
|
|
| Active material (graphite) |
563.6 |
16.4 |
14.1 |
4.3 |
| Binder |
69.7 |
2.0 |
3.1 |
1.0 |
| Current collector (Cu) |
151.9 |
4.4 |
41.6 |
12.8 |
| Cathode (dry) |
|
|
|
|
| Active material |
1,408.6 |
41,0 |
74.4 |
22.9 |
| Carbon |
46.4 |
1.4 |
3.2 |
1.0 |
| Binder |
92.9 |
2.7 |
6.3 |
1.9 |
| Current collector (Al) |
63.0 |
1.8 |
19.4 |
6.0 |
| Electrolyte |
618.0 |
18.0 |
44.0 |
13.5 |
| Separator |
60.5 |
1.8 |
16.4 |
5.0 |
| Rest of Cell |
|
|
|
|
| Tabs, end plates, terminal assemblies |
66.2 |
1.9 |
32.2 |
9.9 |
| Core |
0.9 |
0.0 |
|
|
| Container |
291.0 |
8.5 |
70.1 |
21.6 |
| Total |
3,432.7 |
|
324.8 |
|
|
As an example, the materials costs
for a LiCoO2-based 18650 cell (including
materials processing) can be
estimated at about $1.28 for the entire
cell.15
Materials processing is very difficult
to separate from materials cost
and is therefore included in the materials costs in this section. In addition,
the materials-processing cost changes
dramatically with different materials
and can therefore be considered material-specific. However, new processing
techniques can lower the current high
cost of raw materials.
Manufacturing and Labor Cost
State-of-the-art manufacturing of a
cylindrical cell on a production line includes
mixing and coating, calendaring
and slitting, cutting, winding, tab welding,
automated assembly, and inspection
followed by testing, cycling, and
packaging. To produce 100,000 units
per year requires a total workforce of
76 to 104 people working on two lines
in two shifts. Gaines and Cuenza15 estimated
the labor cost per cell and overhead
costs to be $0.42 based on an
18650 cell.
Total Cost
The total 18650 cell costs add up to
roughly $1.70. Scaling to HEV batteries
results in $1,700 (twice the price
target). There is no set cost target for
EV batteries yet. However, based on
this calculation, one could calculate a
highly uncertain estimate of $17,000
per battery.
The estimate shows that to reach the
goals, a tremendous effort is needed to
reduce processing cost, material cost,
and amount of needed material.
CONCLUSION
There is no doubt that lithium-ion
cell chemistries offer some of the best
options for electrical energy storage for
high-power and high-energy applications
such as transportation and stationary
storage due to their electrochemical
potential, theoretical capacity, and
energy density. However, the estimated
battery cost for the example HEV application
is still twice the price target
established by the USABC and DOE.
With rising oil prices, a slightly higher
price than the target might already receive
enough consumer acceptance for
a successful introduction into the market.
However, the price still has to come
down.
There are clearly needs in the areas
of materials development, optimization,
and processing. The calculations
above separate between materials and
labor costs. However, it is nearly impossible
to separate raw material costs
from material processing costs because
we never use pure raw materials in the
process; rather, we use material compounds
that are suitable for the application
and that are the least expensive in
production. Additionally, even raw materials
and material compounds have
been processed. Thus, new low-cost
processing methods for those materials
and compounds have to be developed
in order to minimize the battery’s “raw
material” cost.
Work is needed on hybrid technologies
such as combining low-cost slurry-based
techniques with treatment methods
to replace tasks that are currently
performed in two different steps. High-speed
treatments, such as radiant processing,
need to be optimized to replace
slow furnace procedures. Investment
costs and manufacturing times need to
be minimized to make them feasible for
battery applications. In addition, hybrid
materials that can perform the functions
of two or more components currently
in use need to be developed and
integrated into batteries (e.g., solid or
high-viscosity electrolytes that do not
need separators, have enhanced lithium
exchange behavior, wet the electrode,
and form a good bond).
ACKNOWLEDGEMENTS
The author gratefully acknowledges
the support from David Howell (Energy
Storage R&D Program Manager, Vehicle
Technologies Program, Office of Energy
Efficiency and Renewable Energy,
Department of Energy) and Raymond
Boeman (Transportation Program Director,
Oak Ridge National Laboratory),
guidance from Craig Blue, and
fruitful discussions with Nancy Dudney
and many other colleagues. This research
at Oak Ridge National Laboratory,
managed by UT-Battelle, LLC, for
the U.S. Department of Energy under
contract DE-AC05-00OR22725, has
been sponsored by the Vehicle Technologies
Program for the Office of Energy
Efficiency and Renewable Energy.
REFERENCES
1. World Batteries, Industry Study with Forecasts to
2010 & 2015 (Study #2095) (Cleveland, OH: Freedonia
Group, 2006).
2. German Federal Ministry of Education and Research,
“Innovation Alliance, Lithium Ion Battery 2015”
(2008), http://www.bmbf.de/de/11828.php.
3. Oak Ridge National Laboratory calculations based
on information from Energy Information Administration,
U.S. Environmental Protection Agency, KEMA,
and University of Delaware (2008).
4. D. Howell, Energy Storage Research and Development,
Annual Progress Report 2006 (Washington,
D.C.: Office of FreedomCAR and Vehicle Technologies,
U.S. Department of Energy, 2007).
5. FreedomCAR and Fuel Partnership and United
States Advanced Battery Consortium, Electrochemical
Energy Storage Technical Team Technology Development
Roadmap (Southfield, MI: USCAR, 2006).
6. D. Howell, Energy Storage Research and Development,
Annual Progress Report 2007 (Washington,
D.C.: Office of Vehicle Technologies, U.S. Department
of Energy, 2008).
7. J. Goodenough, H.D. Abruna, and M.V. Buchanan,
editors, Basic Research Needs for Electrical Energy
Storage (Washington, D.C.: Office of Basic Energy Sciences,
U.S. Department of Energy, 2007).
8. H.A. Kiehne, editor, Battery Technology Handbook,
2nd edition (New York: Marcel Dekker, Inc., 2003).
9. J. Besenhard, editor, Handbook of Battery Materials (Weinheim, Germany: Wiley-VCH, 1999).
10. N.J. Dudney and B.J. Neudecker, “Solid State Thin-
Film Lithium Battery Systems,” Curr. Opin. Solid State
Mat. Sci., 4 (5) (1999), pp. 479–482.
11. A.K. Shukla and T.P. Kumar, “Materials for Next-
Generation Lithium Batteries,” Curr. Sci., 94 (3) (2008),
pp. 314–331.
12. M.S. Whittingham, “Materials Challenges Facing
Electrical Energy Storage,” MRS Bulletin, 33 (4)
(2008), pp. 411–419.
13. J. Newman and C. Monroe, “The Impact of Elastic
Deformation on Deposition Kinetics at Lithium/Polymer
Interfaces,” J. Electrochem. Soc. 152 (2) (2005),
pp. A396–A404.
14. P. Arora and Z. Zhang, “Battery Separators,” Chem.
Rev., 104 (2004), pp. 4419–4462.
15. L. Gaines and R. Cuenza, Costs of Lithium-Ion-Batteries
for Vehicles (Report ANL/ESD-42) (Argonne, IL:
Argonne National Laboratory, 2000).
16. J. Carcone, “Update on Li-ion Batteries” (Paper presented
at the 15th International Seminar and Exhibit
on Primary and Secondary Batteries, Fort Lauderdale,
Florida, 2–5 March 1998).
Claus Daniel is with the Materials Processing
Group, Materials Science and Technology Division,
Oak Ridge National Laboratory, Oak Ridge,
Tennessee and also the Department of Materials
Science and Engineering, University of Tennessee,
Knoxville, TN. Dr. Daniel can be reached at (865)
241-9521; e-mail danielc@ornl.gov.
|