|
Metal matrix syntactic foams are
composites that incorporate hollow
particles in a matrix, where enclosing
porosity inside the thin shell of the
particle leads to low density without
large decreases in mechanical properties.
Studies on Al, Mg, Pb, and
Zn alloy matrix syntactic foams are
available in the published literature. A
large stress plateau region appears in
the compressive stress-strain graphs
of metal matrix syntactic foams. The
height and length of stress plateau can
be tailored by means of particle wall
thickness, volume fraction, and size,
and the total compressive energy absorption
can be controlled. Metal matrix
syntactic foams seem promising
in various energy absorbing applications
including automobile parts since
their energy absorption capability per
unit weight is better than other foams
and lightweight materials.
INTRODUCTION
| HOW WOULD YOU... |
describe the overall significance
of this paper?
This is a review article on
lightweight composite materials
called syntactic foams. Use of
lightweight materials is increasing
in transportation applications
because they can reduce the weight
of the vehicle and improve fuel
economy and reduce pollution. The
paper provides a critical insight
into the progress made in these
materials over the past two decades
and identifies materials that can be
used in such applications.
describe this work to a
materials science and engineering
professional with no experience in
your technical specialty?
In traditional metallurgy, porosity is
an enemy of the casting. However,
innovative methods of enclosing
porosity inside strong hollow
particles and embedding them
inside metals can help in enhancing
several properties of the composite,
while reducing the structural
weight. This review provides an
overview of a large variety of
porous composite materials and
will help in selecting materials for
various applications.
describe this work to a
layperson?
Use of industrial waste fly ash
in creating lightweight materials
that cut down pollution and fuel
consumption of vehicles is a very
attractive idea. This review article
shows these materials absorb a
very high amount of energy under
compression and help in making
automobiles safer.
|
Metal foams are used in lightweight
structures, especially as core materials
in sandwich constructions.1,2 Mechanical
properties of metal foams
are much lower compared to the base
metal, restricting their applications to
where tensile or compressive strength
is not the primary design criterion.3
An innovative method of incorporating
porosity in materials is the use of
hollow particles as fillers. Enclosing
porosity inside stiff and strong shells
and incorporating those shells in matrix
metals leads to porous materials
that have significantly higher modulus
and strength than foams containing
gas porosity. These hollow particle filled materials are called syntactic
foams and are a class of particulate
composites.3,4 Syntactic foams with
over 50 vol.% porosity have been synthesized,
providing substantial weight
saving compared to the matrix material.
Apart from weight reduction, the
presence of controlled size porosity
of a spherical shape with uniform distribution
helps in providing high energy
absorption under compression in
syntactic foams. Therefore, available
studies are mainly related to either
the processing aspects or compressive
property characterization. This review
focuses on the compressive properties
of metal matrix syntactic foams. Numerous
studies are available on other
aspects such as corrosion and electrical
and high strain rate properties,
which are not covered in this review.
Due to the interest in lightweight materials,
most of the published literature
is focused on aluminum alloy matrix
syntactic foams. In addition, developing
lightweight composites of high
density metals such as lead5 and zinc6
is also of interest. Compressive properties
of all these syntactic foams are
compiled and analyzed to find structure
compositionproperty relationships.
HOLLOW PARTICLES
Thin-walled ceramic particles are
beneficial in synthesizing low-density
syntactic foams. Numerous types
of ceramic hollow particles are now
available that can be used for this purpose.
Apart from mechanical property
modification, the use of ceramic hollow
particles provides higher dimensional
stability to the composite by
reducing the thermal expansion coefficient.7 In general, two types of hollow
particles are widely used in synthesizing
syntactic foams.
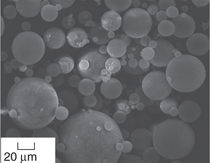
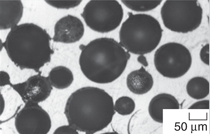
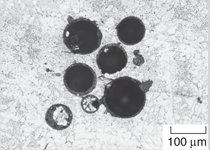
The first type of particles, microballoons,
are high-quality hollow ceramic
microspheres that are commercially
produced and have controlled properties
such as diameter, size distribution,
and wall thickness. Ceramic microballoons
have been used in several studies.811 An example of microballoons
is shown in Figure 1a. These particles
go through several quality control
steps such as pressurization at given
pressure levels to fracture and eliminate
weaker and defective particles,
flotation or air classification to select
only the intact low density particles,
and sieving to obtain given size
ranges. These processing steps ensure
that high-quality particles, with a narrow
distribution of properties, are obtained.
However, these particles are
still not completely free from defects.
Figure 1b shows an example where
porosity and small size solid particles
are embedded inside the wall of a
large microballoon. In addition, some
variation in the wall thickness is also
observed in this broken microballoon.
Such irregularities are not widespread
in glass microballoons. The commercial
microballoons are available in the
density range of about 1001,000 kg/m3. In addition to glass, microballoons
of several ceramics such as silica, alumina,
zirconia, and carbon are also
available now.
The second commonly used particles
are fly ash cenospheres. Fly ash
is produced during coal combustion
and is an industrial waste by-product.
Due to the pozzolanic properties, fly
ash is added to cement and construction
material as its most significant application.12 However, nearly half of the
fly ash produced in the United States
is dumped in landfills. A review article
is available on fly ash, which provides
details on its classification, composition,
and applications.13 One of the
challenges in developing applications
of this waste product is to separate
the useful hollow particles, called cenospheres,
from the coal combustion
by-products, which include a wide
variety of impurities. These processing
methods add cost to this otherwise
freely available material; however,
the cost of fly ash cenospheres is still
much lower than synthetically made
microballoons. Incorporating cenospheres
in metals can lead to substantial
savings on the cost of raw materials
and reduce the pollution that is
generated in the production of metals
like aluminum, which consume a lot
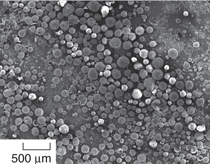
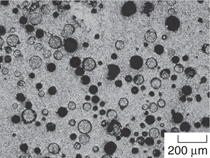
of energy in their production.14 Figure
2a shows a sample of cenospheres
obtained from Trelleborg Offshore
Boston. These processed cenospheres
show a uniform size distribution.
Some of the particles can be defective
as shown in Figure 2b, where porosity,
non-uniform size and shape, and
poor surface finish are among the
defects. Fly ash particles have predominantly
SiO2, Al2O3, and Fe2O3
in their structure.15 An example of a
composition of fly ash obtained from
Wisconsin Electric Power Company in
Milwaukee, in wt.%, is SiO2 61.0%,
Al2O3 25.80%, Fe2O3 4.99%, K2O
3.59%, MgO 1.58%, TiO2 1%,
Na2O 0.74% CaO 0.82%, and SO3
0.31%. Trace amounts of several
toxins may be present in fly ash particles,
including As, Cd, Pb, and Zn,
depending upon the origin of coal and
the combustion reactions.13 Leaching
of these toxins from the particles
is a significant concern, especially in
landfills. Such possibilities should be
considered while developing processing
techniques and applications for cenosphere
filled syntactic foams.
SYNTHESIS METHODS FOR SYNTACTIC FOAMS
Three methods of syntactic foam
synthesis are widely used: pressure
infiltration, stir casting, and powder
metallurgy. These methods have their
advantages and limitations and are selected
based on the material system
and composition.
In pressure infiltration, a preform
or a bed of loosely packed particles
is prepared and placed in a mold.9,16
Molten metal is infiltrated in the mold
by applying either high pressure or
vacuum or a combination of both to
fill the interparticle spaces and form a
near-net-shaped syntactic foam component.17,18 The advantages of this
method include synthesis of foams
containing high volume fraction of
particles (up to 70 vol.%), net-shaped
or near-net-shaped component fabrication,
and low porosity in the composite.
The limitations of this process
include use of high pressure for melt
infiltration leading to fracture of particles,
difficulty in synthesizing syntactic
foams with low particle volume
fraction, and additional cost associated
with preparing a preform. There has been some work on infiltrating
loose beds of cenospheres with molten
alloys to form syntactic foams to eliminate
the need of preparing preforms.
This process requires a very close control
over melt superheat temperature
and particle preheat temperature to
avoid freeze choking of the melt and
incomplete infiltration. Low infiltration
pressure can lead to incomplete
filling of pores and high residual porosity,
whereas high infiltration pressure
can cause particles to fracture or
liquid metal to infiltrate inside the hollow
spaces within the cenospheres due
to defects. Studies have shown effects
of all these processing parameters on
material quality.
In stir casting, the molten melt is
stirred using a high shear impeller
and particles are slowly added in the
vortex formed in the melt.19,20 This
process can be conducted in a conventional
foundry and requires very
little new infrastructure. Low cost and
easy implementation have resulted in
widespread use of stir casting for synthesizing
syntactic foams. Flotation
of low density particles is a concern
in this method, especially when the
particle volume fraction is low. On the
contrary, at high particle volume fractions
high shear processing can lead to
substantial particle fracture. Wetting
of particles with liquid melts is also
a concern. In several studies particles
are coated with suitable materials, including
metals like nickel, to increase
their wettability with the molten melt.
The coating also has the advantage of
sealing the porosity in the hollow microballoons
and cenospheres. For example,
nickel-coated fly ash particles
have been incorporated in aluminum
alloy melts. This method has been
used for fabrication of aluminum alloy
syntactic foams.19,21,22 Use of this
method for lead and zinc matrix syntactic
foams is especially difficult because
of the large density difference
between the particles and the matrix.5
However, the stir casting method can
be followed by slow solidification or
centrifugal casting processes, which
lead to a high concentration of particles
in the top part of the casting.
The top part can be used as the highly
filled syntactic foam, while the bottom
part can be recycled in the subsequent
heats.
Powder metallurgy methods are
used in several studies. These methods
are versatile because a wide variety
of particle volume fractions can be
incorporated in composites. Even reactive
metals, which are not amenable
to liquid state processing, can be used
as the matrix material in the powder
metallurgy method. Hollow particles
and powder of matrix metal are mixed
together in required volume fractions,
followed by compaction and sintering
to obtain syntactic foams.23 However
the powder metallurgy methods have
the disadvantage that fracture of weak
hollow particles can be significant in
the compaction stage at high volume
fractions. This method is especially
suitable in synthesizing syntactic
foams containing low microballoon
volume fraction.
MICROSTRUCTURE
Syntactic foams have a two-component
microstructure, including matrix
material and hollow particles. However,
the microstructure can have several
phases. As the third phase, porosity
entrapped in the matrix alloy can
be significant in some composites and
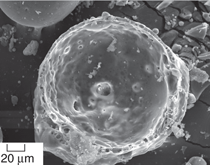
affect the mechanical properties. Figure
3a and b shows microstructures of
two aluminum matrix syntactic foams,
where entrapped air porosity in the
matrix alloy can be observed in the
regions between hollow microspheres.
Additional phases may be present in
the matrix alloy. For example, in magnesium-
aluminum alloys intermetallic
precipitates of Mg17Al12 are present in
the matrix.
Figure 3c shows that the grain size
of the matrix alloy is refined in the
vicinity of the fly ash cenospheres.
A similar effect is observed in magnesium
alloy matrix syntactic foams.
In AZ91 alloy intermetallic precipitates
are present in the matrix along
grain boundaries. The size of these
precipitates is refined by an order of
magnitude in the AZ91/fly ash syntactic
foams as shown in Figure 3d. In
ZC63/fly ash composites, the dendrite
arm spacing was reduced compared to
the matrix alloy cast under the same
conditions. Numerous elements present
in fly ash can diffuse out in the
matrix and lead to grain refinement.
Diffusion of elements from the particle
matrix interface to the interparticle
region depends on the type of element,
concentration, melt temperature, and
processing time.
Apart from the grain size effects, reactions
at the particle-matrix interface
are also observed in several syntactic
foams.24 Interfacial reactions in aluminum
matrix composites can result in
brittle phases that can be detrimental
to the syntactic foam properties. In an aluminum/fly ash system, transmission
electron microscopic analysis
showed the presence of two interpenetrating
crystalline networks in the
matrix comprising a-Al2O3 particles
surrounded by a continuous metallic
network.25 The silicon level was
increased in the matrix in the A356/
fly ash composites as a result of reaction
between the silica of fly ash and
the matrix alloy.22 In magnesium alloy
ZC63/fly ash syntactic foams the main
interfacial reaction product phase was
detected as MgO. These reactions can
be controlled by coating particles with
appropriate metals.
Unlike fly ash cenospheres, commercially
produced glass and ceramic
microballoons have closely controlled
composition and undesired interfacial
reactions are not a major concern with
them. Microballoons can also be coated
with appropriate metals to increase
their wetting characteristics. Nickelcoated
particles have been used for
aluminum and lead alloys.5 In addition,
nickel-coated fly ash cenospheres
have been incorporated in aluminum
alloys.26 It was observed that wetting
characteristics and dispersion of hollow
particles improved as a result of
the coating that was compatible with
the matrix alloy.
COMPRESSIVE PROPERTIES
Stress-strain Graphs
Compressive properties have been
widely studied for a variety of syntactic
foams.27 Representative compressive
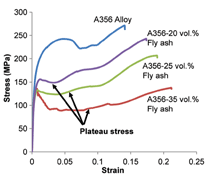
stress-strain curves for A356
alloy matrix syntactic foams are presented
in Figure 4.17 In Figure 4a the
compressive response of the matrix alloy
is compared with syntactic foams
containing different volume fractions
of cenospheres, whereas Figure 4b
presents the effect of cenosphere particle
size on the properties of the composite.
The compressive stress-strain curves
are characterized by a linear region,
followed by a long stress plateau. At
the end of the plateau region stress
starts increasing again. The initial linear
region is normally considered linear
elastic behavior, where modulus
is calculated. However, in syntactic
foams where particles of a wide variety
of wall thicknesses and size are present,
it is not necessary that this region
is truly elastic. Some of the particles
can fail at low stress levels and cause
some variation within this region. Usually
a small stress drop is observed at
the end of the linear region before the
stress plateau appears. Initiation of
cracks in the specimens at the end of
the linear region is usually responsible
for the stress drop. The energy absorption
capabilities of syntactic foams are
mainly related to the height and length
of the stress plateau. Detailed studies
are available in polymer matrix syntactic
foams where the effect of particle
wall thickness and volume fraction on
the strength and energy absorption in
syntactic foams has been studied.28 Although
such systematic studies are not
yet available in metal matrix syntactic
foams, trends similar to those observed
in polymer matrix syntactic foams can
be expected.4 Experimental results have
shown that using thick-walled particles
can increase the strength and modulus
of syntactic foams without causing
significant increase in the density.29,30
The plateau region is where sequential
crushing of microballoons takes
place and the material absorbs energy
without any significant change in the
strength. The particle crushing and
compaction result in the densification
of the composite material. When
the densification is complete, then the
stress starts rising again as visible in
Figure 4a.
Mechanical Properties
Some of the data presented here
have been extracted from the published
graphs. Although precautions
have been taken in image processing,
there may be small variations in the
absolute values. Properties of matrix
alloy are not reported in all studies. In
the absence of matrix properties from
the same study, literature values of the
same composition are taken.
Results obtained from the published
studies on the compressive
properties of syntactic foams are summarized
in Figures 57. The data included
in the graphs correspond to
aluminum,10,17,31,32 magnesium,33,34
titanium,35,36 and zinc6,37 matrix syntactic
foams. Not every study reports
all compressive properties, so the reported
properties are extracted and
included in appropriate graphs. There
are numerous factors that can be controlled
in designing a syntactic foam
microstructure, which include matrix
and particle material, particle wall
thickness, diameter and volume fraction,
and heat treatment of the composite.
These parameters can be used
to minimize the porosity entrapped
in the matrix during the synthesis of
composites and fraction of microballoons
in which liquid metal has entered
and solidified. All these parameters
affect the compressive properties
as well as density of the composite.
Therefore, the comparison presented
in Figures 57 is illustrative of overall
properties of syntactic foams and also
the weight saving potential in selected
applications.
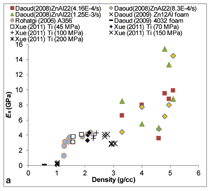
Figure 5a compares the compressive
modulus of syntactic foams over a
large range of density values. Usually
aluminum and magnesium matrix syntactic
foams have the lowest densities,
while titanium and zinc alloy matrix
syntactic foams have higher density
values. The general trend of the data
shows that the higher density syntactic
foams have a higher modulus. The
vertical spread of data for the same
density value shows the possibility of
selecting higher modulus foam having
the same density. In general, the data
are confined within a narrow band and
the choice of different compositions
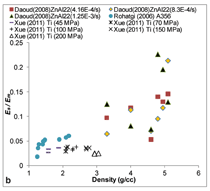
of the same density is small. Figure
5b includes the modulus of syntactic
foam divided by the modulus of its
matrix metal or alloy. This comparison
is more illustrative of the weight saving
potential in syntactic foams comprising
of various matrix materials. It
is observed that the titanium matrix
syntactic foams have higher modulus
for the same density values. However,
the titanium and zinc matrix syntactic
foams have densities over 3 g/cc
and for lower density materials aluminum
and magnesium matrix syntactic
foams appear to be the only options.
The syntactic foam modulus is much
lower than that of the matrix material
due to the porosity present in the
foams.
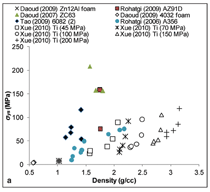
Compressive yield strength of syntactic
foams and the yield strength normalized
with that of the matrix material
are shown in Figure 6. It is possible to
tailor the properties of syntactic foams
over a wide range of strength values
as evident from this figure. It can be
noted in Figure 6b that the strength of
several lightweight syntactic foams is
equal to or close to the strength of the
matrix material. This figure shows that
several low density syntactic foams
can replace their matrix alloys in loadbearing
applications, which can result
in weight saving. In general, higher
density foams show higher strength.
Unlike modulus, strength values are
not confined to a narrow band and
scatter over a wide range for a given
density. Therefore, several compositions
of syntactic foams are available
having the same density but a variety
of strength levels. Data available for
titanium matrix syntactic foams produced
by powder metallurgy method
at different compaction pressures and
microballoon volume fractions is very
illustrative of the change in syntactic
foam properties with density.
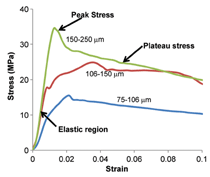
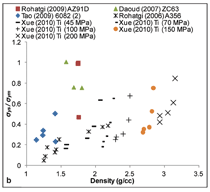
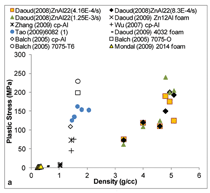
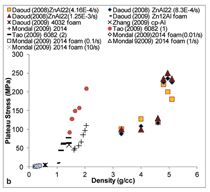
Figure 7 summarizes plastic stress
and plateau stress values of syntactic
foams. In the plastic stress, two different
regions are observed. Plastic
stress of aluminum follows one trend
line, whereas zinc and titanium follow
a different trend line. A wide distribution
of strengths is observed for aluminum
alloy syntactic foams within
a narrow density range. Plateau stress
is an important property of syntactic
foams because it determines the energy
absorption capability of the material.
In general, plateau stress up to
250 MPa is observed in various types
of syntactic foams.
The compressive properties of metal
matrix syntactic foams are strain rate
sensitive.38,39 Evaluation of composite
properties at strain rates relevant to a
given application is required for correct
materials selection. In addition,
in polymer matrix syntactic foams the
failure mode is found to be strain rate
sensitive,40 which should be evaluated
for metal matrix syntactic foams also.
APPLICATIONS
Several present and potential applications
of syntactic foams are discussed
in the available literature. The
damping capacity of 6061Al/fly ash
syntactic foams is found to be much
higher than that of the matrix alloy,
which is beneficial in automotive applications.41 Al-Si alloy/fly ash composites
also showed higher damping
than the matrix alloy.42 Aluminum
matrix syntactic foams have been explored
for making automotive brake
rotors and differential covers.14 Studies
also suggest their use in crash energy
absorption zones.43 Phenolic resins
filled with fly ash have been tested
for automotive break lining applications.44 Metal matrix syntactic foams
can also find similar applications.
Superior wear resistance of A356/fly
ash45 and AA6061/fly ash46 composites
compared to the matrix alloy can
be helpful in such applications. Ni-P/
fly ash,47 Ni-Co/fly ash,47 and Al/fly
ash48 composite coatings have been
used for their wear-resistance properties.
These coatings reduced the wear
of 5083 wrought aluminum alloy.
Electromagnetic (EM) shielding
effects of 2024Al/fly ash composites
were found to be better than the matrix
alloy.49 In the frequency range of
1600 MHz the EM shielding property
of 2024Al alloy was in the range -36
to -46 dB while that of the composites
was in the range of -40 to -102 dB.
These results show suitability of such
composites for lightweight electronic
packaging applications. Nickel coated
fly ash cenospheres have been studied
separately for EM shielding and
microwave absorption applications.50
Syntactic foams of these coated cenospheres
can be effective in electronic
packaging applications.
CONCLUSIONS
Most studies show substantially low
modulus of syntactic foams compared
to the matrix alloy. The strength of
syntactic foams can be nearly equal
to that of the matrix. The plateau and
yield stress can be tailored over a wide
range by selecting appropriate volume
fraction and particle type. Metal
matrix syntactic foams are expected
to have better properties compared
to open or closed-cell metallic foams
since the former have controlled size
and geometry of porosity and the ceramic
shells contribute to stiffness and
strength. Some of the barriers to widespread
use of metal matrix syntactic
foams can be overcome by the availability
of:
- Low-cost defect-free hollow microspheres
in narrow size ranges,
including nanosize ranges
- Mechanical and physical property
data for hollow microspheres
- Mechanical and physical property
data for metal matrix syntactic
foams, especially under high
strain rates
- Processing methods enabling
manufacture of large near-netshaped
parts of syntactic foams
- Understanding of mechanisms of
deformation and fracture, and energy
absorption to develop quantitative
relationships between
structure-processing-property and
predictive capability to design microstructures
- Understanding of solidification
structure formation in the matrix
in the presence of microballoons,
and the influence of microballoons
on grain size, dendrite size,
micro-, and macrosegregation,
and porosity in the matrix
- Understanding the reactions between
the surfaces of microballoons
and molten alloys and developing strategies of preventing
undesirable reactions
Aluminum/fly ash cenosphere composites
have been cast into selected
shapes and their superior properties
have been demonstrated. In certain
cases metal matrix syntactic foams
have been encapsulated in hollow steel
frames for lightweight structures. However,
more research is needed before
metal matrix syntactic foams will be
widely used. Potential of weight saving
in structural applications by using syntactic
foams is evident from the published
data through the graphs plotted
in this review. It is recognized that these
composites may not be suitable where
high modulus is required. However, in
applications where mechanical properties
of these materials are suitable
enough, ability to tailor their properties
is a signifi cant advantage.
ACKNOWLEDGEMENTS
This work is supported by the National
Science Foundation grant
CMMI0726723 and Office of Naval
Research grant N00014-10-1-0988.
The views expressed in the article are
those of the authors, not the funding
agencies. Authors thank William Ricci
at TOB for providing fly ash sample.
REFERENCES
1. P. Colombo and H.P. Degischer, Materials Science
and Technology, 26 (10) (2010), pp. 11451158.
2. J. Banhart, Progress in Materials Science, 46 (6)
(2001), pp. 559632.
3. L.J. Gibson and M.F. Ashby, Cellular Solids:
Structure and Properties (New York: Cambridge
University Press, 1999).
4. M. Kiser, M.Y. He, and F.W. Zok, Acta Materialia, 47
(1999), pp. 26852694.
5. A. Daoud, M.T.A. El-Khair, A.Y. Shenouda, E.
Mohammed, and P.K. Rohatgi, Materials Science and
Engineering: A, 526 (1-2) (2009), pp. 225234.
6. A. Daoud, Materials Science and Engineering: A,
488 (1-2) (2008), pp. 281295.
7. P.K. Rohatgi, N. Gupta, and S. Alaraj, J. Composite
Materials, 40 (13) (2006), pp. 11631174.
8. O. Couteau and D.C. Dunand, Materials Science
and Engineering: A, 488 (1-2) (2008), pp. 573579.
9. D.K. Balch and D.C. Dunand, Acta Materialia, 54 (6)
(2006), pp. 15011511.
10. X.F. Tao, L.P. Zhang, and Y.Y. Zhao, Materials &
Design, 30 (7) (2009), pp. 27322736.
11. M.Y. He, M. Kiser, B. Wu, and F.W. Zok, Mechanics
of Materials, 23 (2) (1996), pp. 133146.
12. N. Chandra, P. Sharma, G.L. Pashkov, E.N.
Voskresenskaya, S.S. Amritphale, and N.S. Baghel,
Waste Management, 28 (10) (2008), pp. 19932002.
13. M. Ahmaruzzaman, Progress in Energy and
Combustion Science, 36 (3) (2010), pp. 327363.
14. P.K. Rohatgi, D. Weiss, and N. Gupta, JOM, 58 (11)
(2006), pp. 7176.
15. B.G. Kutchko and A.G. Kim, Fuel, 85 (17-18)
(2006), pp. 25372544.
16. D.K. Balch, J.G. O'Dwyer, G.R. Davis, C.M. Cady,
G.T. Gray III, and D.C. Dunand, Materials Science and
Engineering A, 391 (1-2) (2005), pp. 408417.
17. P.K. Rohatgi, J.K. Kim, N. Gupta, S. Alaraj, and
A. Daoud, Composites Part A: Applied Science and
Manufacturing, 37 (3) (2006), pp. 430437.
18. L.P. Zhang and Y.Y. Zhao, J. Composite Materials, 41 (17) (2007), pp. 21052117.
19. T.P.D. Rajan, R.M. Pillai, B.C. Pai, K.G.
Satyanarayana, and P.K. Rohatgi, Composites Science
and Technology, 67 (15-16) (2007), pp. 33693377.
20. A. Daoud, M.T. Abou El-khair, M. Abdel-Aziz, and P.
Rohatgi, Composites Science and Technology, 67 (9)
(2007), pp. 18421853.
21. D.P. Mondal, S. Das, N. Ramakrishnan, and K.
Uday Bhasker, Composites Part A: Applied Science
and Manufacturing, 40 (3) (2009), pp. 279288.
22. Sudarshan and M.K. Surappa, Materials Science
and Engineering: A, 480 (1-2) (2008), pp. 117124.
23. M. Hrairi, M. Ahmed, and Y. Nimir, Advanced
Powder Technology, 20 (6) (2009), pp. 548553.
24. R.Q. Guo, D. Venugopalan, and P.K. Rohatgi,
Materials Science and Engineering A, 241 (1-2)
(1998), pp. 184190.
25. N. Sobczak, J. Sobczak, J. Morgiel, and L.
Stobierski, Materials Chemistry and Physics, 81 (2-3)
(2003), pp. 296300.
26. P.K. Rohatgi, R.Q. Guo, H. Iksan, E.J. Borchelt, and
R. Asthana, Materials Science and Engineering: A,
244 (1) (1998), pp. 2230.
27. R.A. Palmer, K. Gao, T.M. Doan, L. Green, and G.
Cavallaro, Materials Science and Engineering: A, 464
(1-2) (2007), pp. 8592.
28. N. Gupta, E. Woldesenbet, and P. Mensah,
Composites Part A: Applied Science and
Manufacturing, 35 (1) (2004), pp. 103111.
29. N. Gupta, R. Ye, and M. Porfiri, Composites Part B:
Engineering, 41 (3) (2010), pp. 236245.
30. M. Porfi ri and N. Gupta, Composites Part B:
Engineering, 40 (2) (2009), pp. 166173.
31. D.P. Mondal, M.D. Goel, and S. Das, Materials
Science and Engineering: A, 507 (1-2) (2009), pp.
102109.
32. X.F. Tao and Y.Y. Zhao, Scripta Materialia, 61 (5)
(2009), pp. 461464.
33. Z.-Q. Huang, S.-R. Yu, and M.-Q. Li, Transactions of
Nonferrous Metals Society of China, 20 (Supplement
2) (2010), pp. s458s462.
34. P.K. Rohatgi, A. Daoud, B.F. Schultz, and T.
Puri, Composites Part A: Applied Science and
Manufacturing, 40 (6-7) (2009), pp. 883896.
35. X.B. Xue, Y.Y. Zhao, V. Kearns, and R.L. Williams.
Supplemental Proceedings: Volume 2: Materials
Characterization, Computation, Modeling and Energy (Warrrendale, PA: TMS, 2010), pp. 129136.
36. X. Xue and Y. Zhao, JOM, 63 (2) (2011), pp. 3640.
37. A. Daoud, Materials Science and Engineering: A,
525 (1-2) (2009), pp. 717.
38. Z.Y. Dou, L.T. Jiang, G.H. Wu, Q. Zhang, Z.Y. Xiu,
and G.Q. Chen, Scripta Materialia, 57 (10) (2007), pp.
945948.
39. D.D. Luong, N. Gupta, and P.K. Rohatgi, JOM, 63
(2) (2011), pp. 4649.
40. V.C. Shunmugasamy, N. Gupta, N.Q. Nguyen, and
P.G. Coelho, Materials Science and Engineering: A, 527 (23) (2010), pp. 61666177.
41. G.H. Wu, Z.Y. Dou, L.T. Jiang, and J.H. Cao,
Materials Letters, 60 (24) (2006), pp. 29452948.
42. Y. Mu, G. Yao, and H. Luo, Materials & Design, 31
(2) (2010), pp. 10071009.
43. Q. Zhang, P.D. Lee, R. Singh, G. Wu, and T.C.
Lindley, Acta Materialia, 57 (10) (2009), pp. 3003
3011.
44. S. Mohanty and Y.P. Chugh, Tribology International,
40 (7) (2007), pp. 12171224.
45. Sudarshan and M.K. Surappa, Wear, 265 (3-4)
(2008), pp. 349360.
46. P.R.S. Kumar, S. Kumaran, T.S. Rao, and S.
Natarajan, Materials Science and Engineering: A, 527
(6) (2010), pp. 15011509.
47. C.N. Panagopoulos, E.P. Georgiou, A. Tsopani,
and L. Piperi, "Composite Ni-Co-Fly Ash Coatings
on 5083 Aluminium Alloy," Applied Surface Science,
doi:10.1016/j.apsusc.2010.10.130.
48. S.P. Sahu, A. Satapathy, A. Patnaik, K.P. Sreekumar,
and P.V. Ananthapadmanabhan, Materials & Design,
31 (3) (2010), pp. 11651173.
49. Z. Dou, G. Wu, X. Huang, D. Sun, and L.
Jiang, Composites Part A: Applied Science and
Manufacturing, 38 (1) (2007), pp. 186191.
50. X.-F. Meng, D.-H. Li, X.-Q. Shen, and W. Liu, Applied
Surface Science, 256 (12) (2010), pp. 37533756.
Pradeep K. Rohatgi, Wisconsin and UWM Distinguished
Professor and Director of UWM Centers of
Composites and Advanced Materials Manufacture,
and Benjamin F. Schultz, postdoctoral research
fellow, are with the Center for Composite Materials,
Materials Engineering Department, University
of Wisconsin-Milwaukee, Milwaukee, WI 53201
USA. Nikhil Gupta, associate professor, and Dung
D. Luong, Ph.D. candidate, are with the Composite
Materials and Mechanics Laboratory, Mechanical
and Aerospace Engineering Department, Polytechnic
Institute of New York University, Brooklyn, NY
11201 USA. Dr. Gupta can be reached at (718) 260-
3080; fax (718) 260-3532; e-mail ngupta@poly.edu. |