Editor’s Note: Sustainability in electronics will be the topic of a webinar on September 27, hosted by the author of this article and co-sponsored by TMS. For more information visit www.tms.org/Meetings/webcast/home.html.
After more than 7,000 years of widespread
use, lead is figuratively sinking
in contemporary industrial ecology and
global societal commerce. But, despite
the long research history of documenting
the detrimental impacts of lead use, and
of legislative initiatives to phase lead out
of various products and processes, the
United States currently has no federal
mandate comparable to the European
Union’s “restriction of the use of certain
hazardous substances in electrical and
electronic equipment” banning the sale
of new electrical and electronic equipment
containing specified levels of six
major toxic materials, including lead.
Without a strong environmental agenda
leaning toward preventive strategies,
concerns about demonstrated public
health effects often prove to be strong
motivators of U.S. materials use policy.
This article assesses various ways in
which universal adoption of lead-free
solders, coupled with additional material
restrictions, may have tangible benefits
for public health and the environment,
and how these benefits may help secure
true innovation in material selection and
product design for the environment.
INTRODUCTION
The Latin word for lead, plumbum,
may have derived from the original
Sanskrit bahu-mala, meaning “very
dirty.”1 Throughout recorded history,
lead has been known to be toxic. H.L.
Needleman2,3 points out that evidence of
lead toxicity existed in 2000 b.c. Having
been phased out of Western civilization’s
staples such as gasoline, paint, and water
distribution pipelines, the elimination of
lead in electronic products is imminent.
The toxic metal’s last major refuge is
automobile batteries which demand more
than 70% of global lead assets but are
largely recycled with notable fugitive
emissions that contaminate the environment
worldwide.4 The events leading to
various legislative initiatives to eliminate
lead from electronic products are based
on its notorious legacy as a major health
hazard across the spectrum of human
generations and cultures.5 However, there
are concerns about anchoring industrial
product design and innovation on legislative
mandates that specify the elimination
of one chemical without providing
rigorous guidelines about the selection of
alternatives which should, in principle, be
less toxic, perhaps cheaper, and certainly function properly in the manufacturing
process in which they are embedded.3
The health effects of human exposure
to lead are better understood than any
potentially viable replacement. As such,
more research is needed to document the
implications of phasing lead out of solder
materials and the likely impacts of the
replacement for human health and the
environment throughout the life cycle
of the materials.
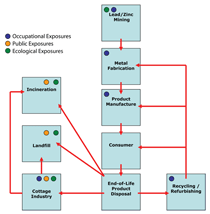
Human exposure to lead occurs at
various stages of production and use as
depicted in Figure 1. Mining of galena
(lead sulfide) represents the origin of the
life-cycle assessment (LCA) of lead, and
this stage has one of the most profound
lasting impacts on environmental quality
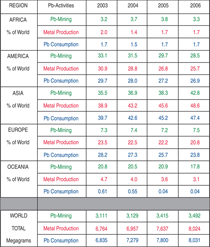
and human health.6,7 Figure 28 presents
data on the trend of lead mining, metal
production, and consumption worldwide.
Clearly, there are some notable
regional disparities in these activities
that are likely to translate to differences
in occupational health risks associated
with mining, metal works, and product
life cycle. The Asia region has dominated
lead mining, production, and consumption
in recent years, and the demand
for lead in that region is growing. The
Americas exhibit a slight decline in lead
mine production, although the United
States harbors some of the strongest
legacies of the adverse effects of lead
mining. Among the best documented
public health effects of lead mines are
associated with the U.S. Environmental
Protection Agency (EPA) Superfund site
at Coeur d’Alene river basin in northern
Idaho. The average blood lead level
(BLL) of children ages 1 through 5 in
the region is 2–3 μg/dL higher than the
national average, and up until the year
2000, more than 15% of the children
had BLL > 10 μg/dL (compared to the
national average of 2%), which is the
action level recommended by the United
States Centers for Disease Control.6
Table I.
Health Effects or Physiological Changes Associated with Blood Lead Levels9
|
Blood Lead Levels (μg/dL) |
| Health Impacts |
Children |
Adults |
| IQ Reduction (1–4 points, mean of 2.6)a |
10–20 |
N/A |
| IQ Reduction (2–5 points, mean of 3.5)a |
20 |
N/A |
| Increased Systolic Blood Pressure (1.25 mm Hg) |
N/A |
10-15b |
| Increased Systolic Blood Pressure (2.50 mm Hg) |
N/A |
15-20b |
| Increased Systolic Blood Pressure (3.75 mm Hg) |
N/A |
Above 20b |
| Gastrointestinal Effects |
60 |
N/A |
| Anemia |
70 |
80 |
| Nephropathy |
80 |
120 |
| Encephalopathy |
90 |
140 |
| aIn children aged 0–1 only; bin men, aged 20–79; NA = not applicable, or data not available. |
|
Cognitive deficits or mild mental
retardation are the most insidious health
hazards associated with lead exposure
in vulnerable children, where a BLL
between 10 μg/dL and 20 μg/dL is associated
with up to 4 points reduction in intelligence
quotient. In adults, hypertension,
anemia, kidney disease, gastrointestinal
disorders, and brain damage are all
documented health hazards associated
with untreated lead poisoning (Table I).9 Despite the introduction of various
legislative initiatives to limit the use of
lead in several industries (see Table II),
most people still have measurable BLL
which is typically less than 5 μg/dL in
the United States, a positive outcome of
phasing lead out of gasoline. Exposure
of the general population to lead comes
from various sources, and it is difficult
to apportion a specific level of risk of
lead exposure to its use in solder materials.
However, the legacy of “hot spots”
including old mines, fugitive releases
from industries, and occupational exposures
may account for most of the current
levels of lead exposure. According to
the U.S. EPA’s Toxic Release Inventory
program, 8.47 million kg of metallic lead
was reported by U.S. industries in 2005,
in addition to 204.38 million kg of lead
compounds, making a total of 212.86
million kg for that year.10 Additional
information on the sources of lead in the
environment can also be derived from
the amount of lead used by the major
industrial sectors. In 2005, 1.46 Mt of
lead was used in the United States, with
88% going to storage batteries and 4.2%
going to ammunition, the largest two
categories. The U.S. Geological Survey
does not routinely disclose the amount
of lead used by electrical and electronic
manufacturing industries (SIC code 36)
to avoid disclosing company proprietary
data.11
LEGISLATIVE INITIATIVES AND MANDATES ON LEAD-FREE ELECTRONICS
Given the legacy of lead as a pervasive
environmental pollutant and the potency
of its health impacts, it is not surprising
that jurisdictions across the world have
tried to limit its use. The current drive
to eliminate lead from solder materials
began with concern over water distribution systems where direct population
exposure to lead from potable water was
demonstrated (Table II). Subsequently,
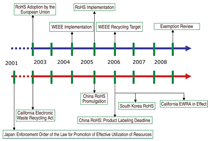
the European Parliament and the Council
of the European Union (EU) on January
23, 2003 adopted directive 2002/95/EC
on the restriction of the use of certain
hazardous substances in electrical and
electronic equipment (restriction of the
use of certain hazardous substances
in electrical and electronic equipment
[RoHS], Figure 3). The directive covered
a broad range of uses for toxic chemicals,
with three articles specifically addressing
lead, as shown in the sidebar.
Table II.
The Fate of Legislative Initiatives to Reduce Lead Use across Various Industrial Sectors in the United States
|
| N. American
Industrial
Classification Systems Codes |
Products |
Pb Component |
Regulartory Program |
Replacement/ Alternative Policy |
Remarks |
| 32411 |
Petrochemical refining |
Tetraethyl Pb additive |
National phase-out 1975-1987 |
Manganese (MMT); MTBE |
Incomplete global phase-out of Pb;
uncertainty about
health and ecological
impacts of alternatives |
| 335911 |
Storage batteries |
Pb electrodes |
State regulated recycling programs |
“Green batteries” nickel-metal hydride |
Voluntary programs, incentives |
| 331511 / 326220 |
Water piping |
|
National phase out June 1986-June 1988 |
“Pb-free” pipes and fittings (8% Pb); “Pb-free solder” (0.2% Pb) |
Fixtures, old tanks remain hazardous for next decade |
| 23321 |
Coloring pigments |
Pb pigments |
Residential Pb-Based Paint Hazard Reduction Act of 1992 (PL 102-550) |
Pb-free pigments |
Persistent litigation; paint replacement hazards in old buildings |
| 334411 |
Electron Tubes |
Pb oxide |
RoHS/WEEE |
Flat panels; Hg |
Premature collection
programs. Int'l
trade. Uncertainty about
Pb leaching conditions |
| 334412 |
Printed circuit boards |
Sn-Pb solders |
RoHS/WEEE |
Pb-free solder, silver, bismuth, indium |
Uncertainty about risks of alternatives; costs and
benefits of switching |
|
After July 1, 2006, the European
RoHS prohibits the sale of electrical
and electronic equipment containing
the four metals listed in the sidebar and
certain brominated flame retardants
at the specified concentrations.12 The
RoHS directive is expected to work in
concert with the Waste Electrical and
Electronic Equipment (WEEE) directive
(2002/96/EC) to reduce the environmental
and human health burden posed by
discarded electronic products.13 Specifically,
WEEE mandates the producers of
electrical and electronic equipment to
finance the collection, recycling, or
otherwise adopt non-hazardous disposal
of their products after consumers are
ready to discard them. Although there
are some exemptions granted or under
review based on the argument that safe
and reliable alternatives are not currently
available,14 most electronics and
electrical equipment manufacturers have
been searching and testing for alternative
materials for more than a decade.
In this regard, much effort has focused
on finding alternatives to the ubiquitous
time-tested tin-lead solder material.5
Following the impetus of the EU directives,
Japan,15 China,16 South Korea,17
and the U.S. state of California18 have
instituted similar regulations to limit the
use of lead and other RoHS toxicants in
electronic and electrical equipment (Figure
3). This convergence of legislative
initiatives has increased the geographical
scope and pace of activities to redesign
electrical and electronic products using
lead-free components. The most vulnerable
component of electronic products
is the printed wiring board, where the
use of eutectic tin-lead solder has been
traditional. Several alternative lead-free
solder materials are now being marketed
and used in electronic products, but in
the absence of clear guidance on performance
reliability, toxicity, and human exposure guidelines in both occupational
settings and the open environment, it has
been difficult to compare the alternatives
to tin-lead solder in terms of potential
for human health impacts. Numerous
lead-free solders have been produced
as replacements. For example, solders
made with various combinations of tins,
silver, and copper (SnAgCu solders) have
been adopted by several Japanese manufacturers
for reflow and wave soldering.
These include SnAg3.0Cu0.5 with a
melting point (m.p.) of 217–220°C;
eutectic SnAg3.5Cu0.9 (m.p. 217°C);
SnZn9 (m.p. 199°C); SnZn8Bi3 (m.p.
191–198°C); SnSb5 (m.p. 232–240°C);
SnAg2.5Cu0.8Sb0.5 (m.p. 217–225°C);
SnIn8.0Ag3.5Bi0.5 (m.p. 197–208°C);
SnBi57Ag1 (m.p. 137–139°C); and
SnIn52 (m.p. 118°C). Whereas annual
tests for BLL are required for workers
using lead, some of the replacement
metals including indium and bismuth
have no clearly established occupational
exposure standards nor do they have
rigorous public health and environmental
regulations associated with their disposal
(Table III).
|
TOXIC CHEMICALS RESTRICTED BY EU LAW
|
Chemicals controlled by the European Union restriction of the use of certain hazardous
substances in electrical and electronic equipment regulations are as follows:
- Mercury in compact fluorescent lamps not exceeding 5 mg per lamp
- Mercury in straight fluorescent lamps for general purposes not exceeding
halophosphate–10 mg; triphosphate with normal lifetime–5 mg; and triphosphate
with long lifetime–8 mg
- Mercury in straight fluorescent lamps for special purposes
- Mercury in other lamps
- Lead in glass of cathode ray tubes, electronic components, and fluorescent tubes
- Lead as an alloying element in steel containing up to 0.35% lead by weight,
aluminum containing up to 0.4% lead by weight, and as a copper alloy containing
up to 4% lead by weight
- Lead in high-melting-temperature-type solders (i.e., tin-lead solder alloys
containing more than 85% lead), specifically, lead in solders for servers, storage,
and storage array systems (exemption granted until 2010); lead in solders for
network infrastructure equipment for switching, signaling, transmission, as well as
network management for telecommunication; and lead in electronic ceramic parts
(e.g., piezoelectronic devices)
- Cadmium plating except for applications banned under Directive 91/338/EEC
amending Directive 76/769/EEC relating to restrictions on the marketing and use of
certain dangerous substances and preparations.
- Hexavalent chromium as an anti-corrosion of the carbon steel cooling system in
absorption refrigerators
|
Even if all electronic products replace
tin-lead solder with lead-free solder and
abide by the RoHS mandate, recent
research indicates that according to California’s
hazardous waste classification
criteria, most electronic products might
still be considered hazardous. This is due to excessive content of copper, nickel,
antimony, and zinc.19 Various organic
chemicals have also been detected, most
of which have no current regulatory
restrictions. These observations make
it clear that there is room for additional
innovation in materials selection and
product design to truly make electronic
products as free as possible from risks to
human health and environmental quality—
beyond the requirement of current
legislative initiatives. The situation is
particularly urgent because of a trade
imbalance that has created a huge market
for used and defunct electronic products
in developing countries. Without a well-developed
recycling and refurbishing
program in developed countries, we
can expect that health risks associated
with outmoded electronic products will
continue to be shifted from one part of
the world to another.20,21 Human exposure
to lead from electronic products is more
problematic because of illegitimate recycling
in cottage industries in developing
countries that have little or no regulatory
oversight.22
International movement of hazardous
products containing lead is under the
regulatory control of The Basel Convention
on the control of transboundary
movements of hazardous wastes and their
disposal.23 The convention entered into
force on May 5, 1992, and as of May
22, 2006, 169 countries have ratified
it. Unfortunately, the United States, a
major source of potentially hazardous
electronic waste, signed in 1990, but has
yet to ratify the convention. Instead, on
March 13 1996, the U.S. government
communicated the following caveats to
the United Nations secretariat regarding
the Basel Convention:
- “It is the understanding of the
United States of America that, as the
Convention does not apply to vessels
and aircraft that are entitled to sovereign
immunity under international
law, in particular to any warship, naval
auxiliary, and other vessels or aircraft
owned or operated by a State and in
use on government, non-commercial
service, each State shall ensure that
such vessels or aircraft act in a manner
consistent with this Convention, so
far as is practicable and reasonable,
by adopting appropriate measures
that do not impair the operations or
operational capabilities of sovereign
immune vessels.
-
It is the understanding of the
United States of America that a State
is a ‘Transit State’ within the meaning
of the Convention only if wastes are
moved, or are planned to be moved,
through its inland waterways, inland
waters, or land territory.
-
It is the understanding of the United
States of America that an exporting
State may decide that it lacks the
capacity to dispose of wastes in an
‘environmentally sound and efficient
manner’ if disposal in the importing
country would be both environmentally
sound and economically efficient.
-
It is the understanding of the United
States of America that article 9 (2) does
not create obligations for the exporting
State with regard to cleanup, beyond
taking such wastes back or otherwise
disposing of them in accordance with
the Convention. Further obligations
may be determined by the parties pursuant
to article 12.
Further, at the time the United States
of America deposits its instrument of
ratification of the Basel Convention,
the United States will formally object
to the declaration of any State which
asserts the right to require its prior
permission or authorization for the
passage of vessels transporting hazardous
wastes while exercising, under
international law, its right of innocent
passage through the territorial sea or
freedom of navigation in an exclusive
economic zone.”
Consequently, it seems certain that
health risks associated with lead-containing
waste electronic products will
continue to flow from the United States
to other countries, particularly because
the United States does not have a national legislation similar to RoHS or WEEE to
restrict the sales of lead-containing products.
Instead of the piecemeal approach
to restricting the disposal of certain
hazardous waste materials into landfills,
a comprehensive review of the bill of
materials in high-volume products such
as cell phones needs to be conducted in
order to target the most toxic components
for replacements. The United States
should also initiate discussion across
states on uniform policies of material
restrictions that will not be confusing
for manufacturers and consumers.
Table III. Comparative Assessment of Environmental
and Health Standards for Metal Use in Solders
|
| Criteria |
Pb
|
Ag
|
Bi
|
Cu
|
In
|
Sn
|
| Permitted Exposure Level, 8 hour-TWA** |
15 mg/m3 |
0.01 mg/m3h |
5 (respirable fraction)– 15 (total dust) mg/m3h |
0.1 (fume)– 1.0 (dust) mg/m3h |
0.1 mg/m3h |
2 (inorganic),0.1 (organic); 5 (respirable fraction) –15 (total tin oxide
dust) mg/m3h |
| Threshold Limit Value***
(mg/m3) |
0.15 |
0.1 |
0.2 mg (Se)/m3 for bismuth selenide; 10 mg/m3
for bismuth telluride |
0.1 |
0.1 |
2.0 |
| Total Maximum Daily Load (Number of Impairments) |
480 |
47 |
No monitoring program |
510 |
No monitoring program |
No monitoring program |
| Maximum Contaminant Level in H2O |
Zero |
0.1 mg/L |
No established standard |
1.3 mg/L |
No established standard |
No established standard |
| Toxic Release Inventory**** |
8.2 (Pb) 170 (Compounds) |
0 04 (Ag) 2.1 (Compounds) |
No monitoring program |
10 (Cu) 630 (Compounds) |
No monitoring program |
No monitoring program |
| Health Impairment Levels |
Blood lead level in children = 10 mg/100 g |
Oral reference dose = 0.005 mg/kg/day |
Man, unreported route: LDLO:221mg/kg; Oral, Rat: LD50: 5g/kg |
Liver storage; 500 mg/kg |
Not established. Indium 111 – in cancer therapy |
Not established standard. |
| Toxicity Symptoms |
Cognitive and development impairment in children; hypertension |
argyria or permanent skin discoloration; tissue degeneration |
"Tellurium breath;" foul breath and stomatitis; malaise, nausea, and depression |
Gastro-intestinal ailment; kidney and liver failure |
No established standard |
Disturbance of immune function; psychosis |
* Bismuth telluride; undoped.
** Occupational Safety and Health Administration.
*** American Conference of Government Industrial Hygienists.
**** National data for year 2000 in million kg. |
|
A NOTE ON "MUSES"
As part of a major programmatic
initiative, the U.S. National Science
Foundation (NSF) launched an interdisciplinary
research agenda titled “biocomplexity
in the environment” (BE).
Biocomplexity refers to “the dynamic
web of often surprising interrelationships
that arise when components of the global
ecosystem—biological, physical, chemical,
and the human dimension—interact.”
Projects supported by the BE
program aim to provide comprehensive
understanding of natural cycles and
processes, the reciprocal effects of
human behaviors and decisions on natural
phenomena, and the ways in which
technological innovation can mediate
the interface between society and nature
in a sustainable way.24 Clearly, the selection and combination of different kinds
of materials used in various products
play a central role in understanding
biocomplexity. Therefore, the NSF created
a sub-program under BE entitled
“Material Use: Science, Engineering and
Society” (MUSES) that is focused on
integrating multidisciplinary research in
engineering, natural sciences, social and
behavioral sciences, economics, mathematics,
and education to address complex
issues related to materials use in
the environment.25
Various academic traditions have
attempted to capture the spirit of MUSES
for some time. The oldest and most
successful of these is “Human Ecology,”
which deals with the interactions
between humans and their natural, social,
and artificial environments. Human
ecology is a multidisciplinary endeavor,
although it has been more closely identified
with the social sciences. Generations
of scholars have made their careers in
human ecology, and there is at least one
major scholarly journal.26 An attempt to
integrate environmental policy, political
systems, and behavior more closely
with human ecology to make it an active
rather than descriptive endeavor led to
the creation of another multidisciplinary
program called “Social Ecology.” The
boundaries between human ecology
and social ecology are permeable, but
there is an Institute of Social Ecology,
which published “Harbinger: a journal
of social ecology.”27 In the early 1990s,
a more focused conceptual framework to
analyze the relationships between human
and natural systems emerged through the
definition of “Industrial Ecology,” which
attempted to model industrial systems to
fit more closely with natural ecosystems
that are essentially closed systems with
the input of energy from the sun, and
no waste products. Industrial ecology
deliberately incorporates an international
dimension to the understanding of human
and social ecology, and it is perhaps more
analytical than philosophical in its orientation.
There is a strong methodological
framework heavily invested in materials
life-cycle assessments. There is a training
component nurtured by the International
Society for Industrial Ecology,28 and
there is a Journal of Industrial Ecology published by MIT Press.29
All these academic traditions approach
the same urgent question through different
pathways: how do we find innovative
solutions to environmental problems
without compromising economic productivity
and human and ecological well
being? The issue of lead-free solders is an
excellent example of a challenging problem
for society, regardless of whether
you approach it as a human ecologist, a
social ecologist, an industrial ecologist,
or simply as a materials scientist.
ACKNOWLEDGEMENT
Research in the author’s laboratory
is supported in part by grants from the
National Science Foundation (DMI-
0223894 and CMS-0524903) and by
an interdisciplinary research award
TS-30856 from the University of California
Toxic Substances Research and
Teaching Program. Additional support
was provided by the Program in
Industrial Ecology at the University of
California-Irvine. I thank my colleagues,
Jean-Daniel Saphores, Julie Schoenung,
Andrew Shapiro, and our students and
postdoctoral researchers for helpful
discussions on this project.
REFERENCES
1. P. van der Krogt, “Elementymology and Elements
Multidict.” http://elements.vanderkrogt.net/elem/pb.html (accessed 04-02-07).
2. H.L. Needleman, “History of Lead Poisoning in the
World,” Lead Poisoning Prevention and Treatment:
Implementing a National Program in Developing
Countries, ed. A.M. George (Bangalore, India: The
George Foundation, 1999).
3. H.L. Needleman, “Low Level Lead Exposure and
Neuropsychological Performance,” Lead Versus
Wealth, ed. M. Rutter and R.R. Jones (New York: John
Wiley and Sons Ltd., 1983), pp. 229–242.
4. Blacksmith Institute, “The World’s Worst Polluted
Places,” www.blacksmithinstitute.org (accessed 04-02-
07).
5. J.M. Schoenung et al., “Adopting Lead-Free
Electronics: Knowledge Gaps and Policy Differences,”
Journal of Industrial Ecology, 8 (2005), pp. 59–85.
6. Superfund and Mining Megasites: Lessons from the
Coeur d’Arlene River Basin (Washington, DC: National
Research Council, National Academy Press, 2005).
7. Analysis Plan for Human Health and Ecological Risk
Assessment for the Review of Lead National Ambient
Air Quality Standards (Research Triangle Park, NC:
United States Environmental Protection Agency, Office
of Air Quality Planning Standards, 2006), www.epa.gov/ttnnaaqs/standards/pb/data/pb_analysis_plan_053106.pdf (accessed 04-02-07).
8. International Lead Zinc Study Group, www.ilzsg .org/ilzsgframe.htm (accessed 04-02-07).
9. A. Pruss-Ustun et al., “Lead Exposure,” Comparative
Quantification of Health Risks: Global and Regional
Burden of Disease Attributable to Selected Major Risk
Factors, ed. M. Ezzati et al. (Geneva, Switzerland:
World Health Organization, 2004), pp. 1495–1552.
10. United States Environmental Protection Agency,
“Toxics Release Inventory Explorer,” www.epa.gov/triexplorer/ (accessed on 04-03-07).
11. P.N. Gabby, 2005 Minerals Year Book: Lead (Reston, VA: U.S. Department of the Interior/Geological
Survey, 2007), http://minerals.er.usgs.gov/minerals/pubs/commodity/lead/leadmyb05.pdf (accessed 04-
03-07).
12. European Union (Brussels, Belgium), “RoHS
Compliance in the EU,” www.rohs.eu/english/index.html (accessed 04-03-07).
13. European Union (Brussels, Belgium), “Waste
Electrical and Electronic Equipment,” http://ec.europa.eu/environment/waste/weee/index_en.htm (accessed
04-03-07).
14. A.A. Shapiro et al., “Implications of Lead-Free
Microelectronics Assembly in Aerospace Applications,”
IEEE Transactions Components and Packaging
Technologies, 29 (2006), pp. 60–70.
15. Government of Japan; Ministry of Economy, Trade,
and Industry, “Law for the Promotion of Effective
Utilization of Resources,” www.meti.go.jp/policy/recycle/main/english/law/promotion.html (accessed
04-03-07).
16. J.H. Lau and L.D. Jun, “Key Differences between
EU RoHS and China RoHS (As of August 7, 2006),”
Global SMT & Packaging, 6 (9) (2006), pp. 10–13.
17. Ministry of Environment, Republic of Korea, “Korea
RoHS,” http://www.korearohs.com/ and http://eng.me.go.kr/docs/index.html (accessed 04-03-07).
18. California Department of Toxic Substances
(Sacramento, CA), “Electronic Hazardous Waste,”
www.dtsc.ca.gov/HazardousWaste/EWaste/(accessed 04-03-07).
19. J.D. Lincoln et al., “Leaching Assessments of
Hazardous Materials in Cellular Telephones,”
Environmental Science & Technology, 41 (2007), pp.
2572–2578.
20. J.-D. Saphores et al., “Household Willingness to
Recycle Electronic Waste: An Application to California,”
Environment and Behavior, 38 (2006), pp. 183–208.
21. J.-D. Saphores et al., “California Households’
Willingness to Pay for “Green” Electronics,” Journal of
Environmental Planning and Management, 50 (2007),
pp. 113–133.
22. A.O.W. Leung et al., “Spatial Distribution of
Polybrominated Diphenyl Ethers and Polychlorinated
Dibenzo-p-dioxins and Dibenzofurans in Soil and
Combusted Residue at Guiyu, An Electronic Waste
Recycling Site in Southeast China,” Environmental
Science & Technology, 41 (8) (2007), pp. 2730–2737,
DOI: 10.1021/es0625935.
23. “The Basel Convention on the Control of
Transboundary Movements of Hazardous Wastes
and their Disposal” (Chatelaine, Switzerland: United
Nations Environment Program, 2002), www.basel.int/ .
24. National Science Foundation (Arlington, VA),
“Biocomplexity in the Environment,” www.nsf.gov/geo/ere/ereweb/fund-biocomplex.cfm (accessed on 04-
23-07).
25. National Science Foundation (Arlington, VA),
“Materials Use: Science Engineering, and Society
(MUSES),” www.nsf.gov/funding/pgm_summ.jsp?pims _id=13654&org=ENG&from=home (accessed on 04-
23-07).
26. Human Ecology: An Interdisciplinary Journal
(Netherlands: Springer), www.springerlink.com/content/1572-9915/ (accessed on 04-23-07).
27. M. Bookchin, “An Overview of the Roots of Social
Ecology,” Harbinger: A Journal of Social Ecology, 3
(2003), pp. 6–11.
28. International Society for Industrial Ecology (Yale
University, New Haven, CT), www.yale.edu/isie (accessed on 04-23-07).
29. Reid Lifset, editor, Journal of Industrial Ecology,
www.mitpressjournals.org/loi/jiec (accessed on 04-23-
07).
Oladele A. Ogunseitan, Professor of Public Health
and Professor of Social Ecology, Director, Industrial
Ecology Research Group, University of California,
Irvine, CA 92697-7070 USA; (949) 824-6350; fax (949)
824-2056; e-mail Oladele.Ogunseitan@uci.edu
|