|
Engine designers show continued
interest in titanium aluminides based
on the intermetallic γ-TiAl phase as
lightweight structural materials to be
used at moderately elevated temperatures.
Although alloy development has
made significant progress in terms of
mechanical properties and environmental
resistance, protective coatings have
been developed that help to extend the
lifetime of these alloys significantly. The
major challenge of coating development
is long-term stability of a protective oxide
scale that forms during service for which
purpose alumina formation is essential.
Furthermore, changes of coating chemistries
at high temperatures must be
controlled to avoid rapid degradation of
the coatings due to diffusional losses into
the substrate material and vice versa.
INTRODUCTION
Titanium aluminide alloys based on
γ-TiAl have convincingly demonstrated
that they can meet the requirements for
automotive combustion engines as well
as for aero engines.1,2 Titanium aluminides
are already in use in small-series
automotive applications. However, in
some cases the risk and cost issues of
γ-TiAl implementation into aero engines,
for example as high-pressure compressors
or low-pressure turbine airfoils (see
Figure 1), are seen as higher than tolerable
in current engine programs,1 thus
preventing these alloys from aero-engine
application. Nevertheless, the potential
of titanium aluminides is indisputable3
and has spurred R&D activities worldwide.
Recent developments indicate
that the past problems related to the
supply chain of γ-TiAl aero-engine parts
might have been overcome, considerably enhancing the trust of designers
in intermetallics. Therefore, today it
appears more likely than ever that aero
engines will be equipped with γ-TiAl
parts in the near future.
At the same time, materials and technology
development continues. Alloy
development aims at increased temperature
capabilities up to temperatures in
excess of 900°C. Although γ-TiAl alloys
possess better intrinsic resistance against
environmental attack than, for example,
conventional titanium alloys, protective
coatings are required for both environmental
protection and thermal insulation
of the structural material when targeting
higher temperatures. Even third-generation
γ-TiAl alloys such as Ti-45Al-8Nb
suffer from mixed oxide scale formation
which results in unacceptably high oxidation
rates and short lifetimes. Despite the
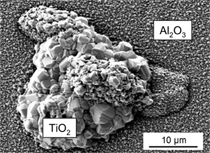
high aluminum content of γ-TiAl alloys,
they do not exclusively form protective
alumina (Al2O3) scales but always build
titania (TiO2) as well (Figure 2), which
is a fast-growing oxide that does not
provide long-term oxidation protection.
Therefore, coatings have to be developed
that form and maintain protective alumina
scales over their entire anticipated
lifetime.
In this study, metallic and nitride
overlay coatings produced by different
magnetron sputtering techniques
were investigated. The coatings were
deposited onto third-generation titanium
aluminide alloy Ti-45Al-8Nb to
improve its environmental resistance.
Further, thermal barrier coatings (TBCs)
deposited by electron-beam physical vapor
deposition (EBPVD), which are
typically deposited onto nickel-based
superalloys, were tested on γ-TiAl.
In addition to the sputtered metallic
and nitride overlay coatings, diffusion
aluminide coatings were used as bond
coats for TBC application.
|
EXPERIMENTAL PROCEDURES
|
Extruded gamma titanium aluminide alloy Ti-45Al-8Nb (in at.%) provided by GKSS
in Germany was used as a substrate material. Specimens were 15 mm in diameter and
1 mm in thickness; the surfaces were ground with SiC paper up to 2,500 grit. Prior to
coating deposition, specimens were cleaned ultrasonically using an industrial cleaning
procedure.
An industrially sized HTC 1000-44 coating unit installed at Sheffield Hallam University
was used to deposit Ti-Al-Cr-Y-N coatings. Prior to coating deposition, the substrate
surfaces were subjected to an intensive metal ion bombardment. In this step, Cr+ ions
(average charge state of 2.1) are generated from steered arc discharge sustained on one
chromium target. The ions are then accelerated to the substrate by the applied high bias
voltage (Ub= –1,200 V). The energy acquired by the metal ions (EI~2.5 keV) is sufficient
for intensive sputter cleaning of the substrate surface and, more importantly, to produce
low-energy implantation combined with radiation damage enhanced diffusion.5,6 The
microstructure of the Ti-Al-Cr-Y-N coatings is single-phase NaCl. Details on the coating
process are given in Reference 7. After nitride coating deposition, the coating process
was concluded by deposition of a thin oxy-nitride layer as described elsewhere.8 Overall
coating thickness was 4 μm.
In addition, metallic Ti-Al-Cr coatings produced by magnetron sputtering in a
laboratory coater at DLR, Institute of Materials Research,9,10 were included in this study.
The ternary coatings were deposited in a dual source using one elemental chromium
target and one compound target, the latter consisting of a titanium disk and cylindrical
aluminum inserts. Coatings were deposited while the specimens were rotated in the
center of the two targets. Coating thickness ranged between 20 µm and 30 µm.
Thermal barrier coatings (7 wt.% partially yttria stabilized zirconia) were deposited
using a 150 kW electron-beam physical-vapor deposition coater installed at DLR; coating
thickness was 170–190 μm. Prior to thermal barrier coating deposition, the substrates
were pre-oxidized to form an alumina scale.
Cyclic oxidation tests were performed in automated rigs in air in the temperature
range of 750°C to 950°C. One cycle consisted of 1 h at temperature and 10 min. cooling
down to 70°C. Specimens were weighed and visually inspected before exposure and
during testing up to a total exposure time of 3,000 h.
Post-oxidation investigations described in this paper were performed using a LEO
Gemini field-emission gun scanning-electron microscope equipped with an Oxford
energy-dispersive x-ray spectrometry (EDS) detector attached. Elemental compositions
were determined using semi-quantitative analysis for spot and line scan measurements.
The EDS system was calibrated with a cobalt standard that was measured immediately
before each session. The EDS software then used the measured cobalt standard for
internal calibration of all elements.
|
OXIDATION-RESISTANT COATINGS
Metallic Coatings
Alloys from the Ti-Al-Cr system
were reported to have good oxidation resistance combined with reasonable
mechanical properties when compositions
are chosen from the two-phase
region γ-TiAl and Ti(Cr, Al)2.11 When
applied as coatings to Ti-45Al-8Nb
by magnetron sputtering,12 Ti-Al-Cr
coatings provide excellent oxidation
resistance at 750°C13,14 for up to several
thousand hours. Compared with the
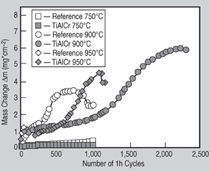
uncoated reference material, at 900°C,
the Ti-Al-Cr coatings improve oxidation
resistance up to about 1,000 1-h cycles
before rapid oxide scale formation occurs
(Figure 3). At 950°C, where the oxide
scale on the uncoated reference sample
readily spalls after a few cycles, Ti-Al-Cr
coatings provide reasonable protection
for up to 350 1-h cycles before breakaway
oxidation occurs. Good oxidation
resistance of the Ti-Al-Cr coatings at
temperatures between 750°C and 900°C
is obviously caused by the two-phase
microstructure of the coating with
the Laves phase being most oxidation
resistant. Scanning-electron microscopy
(SEM) cross-section analysis after 2,000
h oxidation at 750°C reveals that basically
the initial microstructure consisting
of γ-TiAl and Laves phase is maintained
(Figure 4). Compositions of the different
phases are given in Table I.
The oxide scale formed under these
conditions has a thickness of about 2 μm
and consists of a continuous alumina
layer providing excellent protection. An
Oxford energy-dispersive x-ray spectrometry
(EDS) cross-section analysis
revealed14 that in addition to alumina the
outer oxide scale contained some TiO2
and Cr2O3. However, alumina was clearly
the dominating oxide phase (see Table I).
The interdiffusion zone is characterized
by a zone depleted in Laves phase with the remainder being γ-TiAl and a more
or less continuous zone of Laves phase
interface precipitates that formed due
to chromium inward diffusion from the
coating into the substrate alloy. Neither
cracks nor pores were observed in the
interdiffusion zone.
Experimental results clearly indicate
that the sputtered Ti-Al-Cr coatings can
improve oxidation resistance of γ-TiAl
alloy Ti-45Al-8Nb up to a maximum
temperature somewhat below 900°C.
At higher temperatures, the coatings
tend toward strong interdiffusion with
the substrate alloy, which obviously
promotes the formation of less protective
oxide scales. Once the continuous
alumina scale is interrupted, titanium
oxide rapidly forms and controls the
rate of oxide scale growth (breakaway
oxidation). Future Ti-Al-Cr coating
development will consider this issue
more stringently.
Nitride Coatings
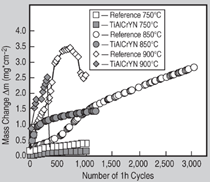
Ti-Al-Cr-Y-N coatings have excellent
oxidation resistance at 750°C15 and
effectively reduce the mass gain caused
by oxide scale formation by a factor of
four compared to the uncoated reference
material (Figure 5, reference time: 1,000
h). Scanning-electron microscopy/EDS
investigations of the oxide scale revealed
a duplex oxide scale consisting of an
outer layered zone with alternating
enrichment of Al2O3 and TiO2 and a
mixed titania-alumina inner layer (Figure
6). At 750°C, the long-term stability of
the Ti-Al-Cr-Y-N coating was proven
up to 3,000 h in isothermal tests.12 The
oxidized surface of the nitride coating
was fairly porous. Few agglomerations
of oxides were observed. Chromium and
yttrium were also detected in the oxide
scale, however, at relatively small levels
(Cr ≤ 1.5 at.%, Y ≤ 0.8 at.%). Yttrium
was not found in the outer part of the
oxide scale. Oxygen was not detected
in and beyond the remaining nitride
coating, which exhibited columnar
grain morphology.12 Scanning-electron
microscopy studies12 revealed a base
layer at the lower end of the nitride
coating. The initial composition of this
layer was different from that of the protective
coating. In the transition zone,
the titanium concentration increased
whereas aluminum was depleted. The
concentration of nitrogen decreased
toward the substrate, revealing an intermediate
plateau near the coating layer.
The enrichment of titanium was related
to the formation of titanium nitride.
With decreasing nitrogen and increasing
aluminum content, Ti2AlN might be
formed adjacent to the substrate.
At 850°C, the nitride coating still provided
good oxidation resistance despite
the fact that it experienced relatively high
initial mass gain during the first 100 1-h cycles (Figure 5). Once a protective
oxide scale formed, the oxidation rate
dropped significantly. Clearly, the oxidation
curve of the Ti-Al-Cr-Y-N-coated
Ti-45Al-8Nb sample falls below that of
the uncoated reference after 1,200 1-h
cycles. At 900°C, the nitride coatings failed after about 300 1-h cycles by oxide
scale spallation (Figure 5). Post-oxidation
SEM investigations revealed that at
this point the entire nitride layer was oxidized.
From these results the maximum
useful temperature is concluded to be
850°C for the Ti-Al-Cr-Y-N coating.
It can be concluded from these and
earlier results that protective Ti-Al-Cr
and Ti-Al-Cr-Y-N coatings have been
developed which might be useful to
protect γ-TiAl components up to around
900°C. Future coating development
aims at further improvement of the
high-temperature capability and lifetime
extension of the coated component.
As a note, quite obviously coatings
add costs to a fully finished component
which must be considered. With this in
mind, the authors have chosen industrially
available processing methods that are
already widely used in industry (partly
even in the aero-engine industry), thus
keeping the additional costs for coating
deposition at a reasonable level.
However, once coatings have matured,
the lifetime benefit and the allowable
increase in service temperature provided
by the use of environmentally resistant
coatings are expected to pay off fairly
quickly. Even if the service temperature
can be increased only by a few 10K,
this might be enough to replace heavier
nickel-based superalloys, which results
in significant weight reduction.
THERMAL BARRIER COATINGS
Thermal barrier coatings have been
widely used on gas turbine hardware to
insulate thermally loaded nickel-based
superalloy airfoils.16–21 This technology
was recently successfully applied to γ-TiAl-based alloys by the present
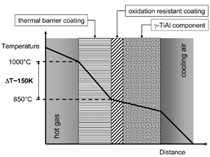
authors.12,13 The concept of TBCs is illustrated
in Figure 7. Due to the presence
of a ceramic coating with low thermal
conductivity (typically yttria-stabilized
zirconia), a temperature gradient is
established across the wall thickness of
an internally cooled component such as
a turbine blade or an actively cooled flat
structure. As the schematic temperature
profile suggests, in the presence of such
a TBC, the useful surface temperature of
the component can be increased by up to
150K, depending on the thickness and
the thermal conductivity of the ceramic
coating, while the metal temperature
remains low. Since the lifetime or the
temperature limit of a γ-TiAl component
is largely determined by its oxidation
resistance and/or its mechanical properties
at elevated temperatures, the use
of TBCs on γ-TiAl might further push
the useful service temperature limit of
γ-TiAl components into a range which
is yet dominated by superalloys.
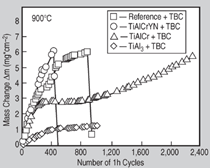
Figure 8 shows mass change curves
of four different TBC systems on γ-TiAl
alloy Ti-45Al-8Nb exposed to cyclic
testing at 900°C. The reference system
(pre-oxidized Ti-45Al-8Nb substrate
plus TBC top coating) shows reasonable
performance up to a total number of 950
1-h cycles. Except for some edge-chipping
phenomena leading to occasional,
small irregularities in the mass change
curve, the TBC was well adherent to the
substrate but then failed by sudden spallation.
As known from earlier work,12–14
TBCs on γ-TiAl alloys can accept the formation
of fairly thick oxide scale (20–30
µm) without failure, which is much more
than allowable for TBC-coated nickel based
superalloys; the critical oxide scale
thickness for the onset of TBC spallation
is typically reported to be about 7 μm for
nickel-based superalloys.20 The current understanding for TBC on nickel-based
alloys is that once the thermally grown
oxide scale has reached a critical thickness,
the stresses generated in this thin
oxide layer during formation and growth
(typically 3–4 GPa20) promote spallation
of the oxide scale, and along with it goes
the TBC. Stress generation in the oxide
scale is mainly due to growth stresses
(approximately 1 GPa in typical TBC
systems) within the oxide scale and due
to the differences in the coefficients of
thermal expansion between the oxide
scale grown at high temperatures and
the metal substrate.
Growth stresses have not yet been
measured for thermally grown oxide
scales on TBC-coated γ-TiAl alloys, but
the main reason for good TBC adherence
to these intermetallics is thought
to be the lower mismatch in thermal
expansion coefficient compared with
nickel-based superalloys, which leads
to lower stresses during thermal cycling.
Therefore, thicker oxide scales can be
developed on γ-TiAl alloys before oxide
scale (and TBC) spallation occurs.
The lowest mass change was measured
for an aluminide-coated TBC system on
Ti-45Al-8Nb (Figure 8, TiAl3+TBC)
which was tested up to 1,000 1-h
cycles. Processing of diffusion aluminide
coatings is crucial with regards to
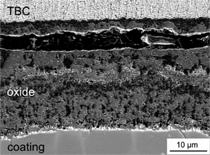
performance. Figure 9a shows a cross
section SEM micrograph indicating
rapid oxidation of a poorly processed
aluminide coating acting as a bond coat
for an EB-PVD thermal barrier top coating.
After 1,000 1-h cycles, a massive
oxide scale was formed with a complex
oxide composition and a porous microstructure.
Failure of the TBC occurred
within the oxide scale along an area
with highest porosity (see the gap in
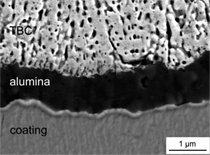
Figure 9a). In contrast to that, the oxide
scale formed on a properly processed
TiAl3 coating is very thin (1 μm after
1,000 1-h cycles, see Figure 9b). The
oxide scale formed is alumina, which
contains some porosity. The TBC is well
attached to the alumina scale, which has
good adherence to the TiAl substrate.
Aluminide coatings provide excellent
oxidation protection, but their inherent
brittleness is considered crucial in practical
applications. Even without external
mechanical loading these coatings tend
toward crack formation just through
stresses induced by thermal mismatch
between the coating and the substrate.
The mass change curves in Figure 8
indicate that, at 900°C, the metallic Ti-Al-Cr coating provides reasonable
protection while the Ti-Al-Cr-Y-N tends
toward rapid oxidation, leading to early
TBC spallation. This is in general agreement
with the findings discussed previously
for these coating systems. For the
Ti-Al-Cr-coated TBC, mass change
exhibits a plateau of slow oxide growth
between 200 and 1,000 1-h cycles and,
thereafter, increases significantly, finally
leading to breakaway oxidation and
spallation. While after 120 1-h cycles at
900°C the oxide scale formed underneath
the ceramic top coating is continuous,
the Ti-Al-Cr coating is already depleted
in Laves phase and substantial interdiffusion
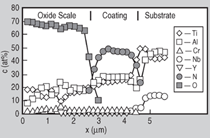
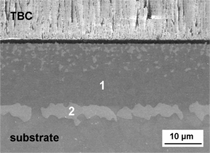
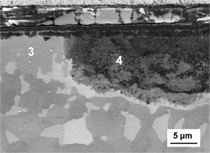
with the substrate has occurred (see markers 1 and 2 in Figure 10a). At
950°C, which is also representative for
longer exposure times at 900°C, the
coating is locally strongly oxidized
which leads to considerable mass gain
(Figures 8 and 10b). Longer exposure
or higher temperatures lead to aluminum
depletion below the oxide scale, resulting
in the formation of fast-growing
non-protective titania (Figure 10b, markers
3 and 4).
Current work on TBCs for titanium
aluminides indicates significant potential
for practical application on aero engine
hardware. However, the oxidation issue
of the TiAl structural material must be
overcome, possibly by the use of protective
coatings that provide long-term
stability of a protective alumina scale
which serves as a bond coat between the
substrate and the TBC. Finally, the
internal cooling of components such as
airfoils marks a potentially insurmountable
challenge due to the inherent brittleness
of titanium aluminides. However,
once titanium aluminides have made
their way into aero engines as airfoils,
flat structures such as casings and cones
might follow. Some of these structures could be actively cooled from the backside,
making use of TBCs desirable.
CONCLUSIONS
Metallic Ti-Al-Cr and ceramic Ti-Al-Cr-Y-N coatings provide good oxidation
protection to intermetallic γ-TiAl alloys.
Yet their long-term stability is insufficient at temperatures around and beyond
900°C, both with regards to maintenance
of a protective oxide scale as well as
their tendency for interdiffusion with the
substrate material. Recent work on TBCs
on intermetallic titanium aluminides has
proven significant potential for actively
cooled turbine hardware. The major challenge
is improved oxidation resistance
of the substrate material. Oxidation resistant
coatings with long-term ability
to form protective alumina might serve
as bond coats that provide good adherence
of the ceramic top coatings to the
substrate, which is particularly needed
under thermal cyclic conditions.
ACKNOWLEDGEMENTS
Part of this work is continued and
extended in the European project
INNOVATIAL–Innovative processes
and materials to synthesize knowledge based
ultra-performance nanostructured
PVD thin films on gamma titanium
aluminides, which is supported by the
European Commission within the Sixth
Framework Programme for Research
and Technological Development.
REFERENCES
1. A. Gilchrist and T.M. Pollock, Struct. Intermetallics 2001, ed. K.J. Hemker et al. (Warrendale, PA: TMS, 2001), pp. 3–12.
2. W. Smarlsy et al., Struct. Intermetallics 2001, in Ref. 1, pp. 25–34.
3. N.A. Walker and N.E. Glover, Struct. Intermetallics 2001, in Ref. 1, pp. 19–24.
4. W.D. Münz, D. Schulze, and F.J.M. Hauzer, Surf. Coat. Tech., 50 (1992), p. 169.
5. G. Håkansson et al., Surf. Coat. Tech., 48 (1991), p. 51.
6. I. Petrov et al., Thin Solid Films, 302 (1997), p. 179.
7. C. Leyens et al., Surf. Coat. Tech., 155 (2-3) (2003), pp. 103–111.
8. M.I. Lembke et al., Surf. Eng., 17 (2) (2001), pp. 153–158.
9. C. Leyens, M. Peters, and W.A. Kaysser, Adv. Eng. Mat., 2 (5) (2000), pp. 265–269.
10. C. Leyens et al., Surf. Coat. Tech., 108-109 (1998), pp. 30–35.
11. M.P. Brady et al., JOM, 48 (11) (1996), pp. 46–50.
12. C. Leyens et al., Gamma Titanium Aluminides 2003, ed. Y.-W. Kim, H. Clemens, and A.H. Rosenberger (Warrendale, PA: TMS, 2003), pp. 551–557.
13. C. Leyens, R. Braun, and M. Peters, 10th World Conference on Titanium (Hamburg, Germany: Wiley-VCH, 2003).
14. C. Leyens and R. Braun, Mater. Sci. Forum, 461-464 (2004), pp. 223–230.
15. C. Leyens et al., Materials for Advanced Power
Engineering, ed. J. Lecomte-Beckers et al. (Juelich,
Germany: Schriften des Forschungszentrum, Reihe
Energietechnik, 2002), pp. 465–474.
16. T.N. Rhys-Jones and F.C. Toriz, High Temp. Technol., 7 (2) (1989) pp. 73–81.
17. R.A. Miller, “Thermal Barrier Coatings for Aircraft Engines–History and Directions,” in NASA CP 3312 (1995), pp. 17–34.
18. M.F. Trubelja et al., Advanced Turbine Systems Annual Program Review Meeting 1997 Conference
Proceedings (Washington, D.C.: U.S. Department of Energy).
19. D.V. Rigney et al., J. Therm. Spray Tech., 6 (2) (1997) pp. 167–175.
20. C. Leyens et al., Zeitschrift fur Metallkunde 92 (7)(2001) pp. 762–772.
21. M. Peters et al., Adv. Eng. Mater. 3 (4) (2001) pp. 193–204.
C. Leyens holds the Chair of Physical Metallurgy
and Materials Technology at the Technical
University of Brandenburg at Cottbus, Germany,
and is associate director of the Institute of
Materials Research at the German Aerospace
Center (DLR), Cologne, Germany. R. Braun and
M. Fröhlich are with the Institute of Materials
Research at DLR. P. Eh. Hovsepian is head of
the Nanotechnology Center for PVD Research at
Sheffield Hallam University, United Kingdom.
For more information, contact Christoph Leyens,
Technical University of Brandenburg at Cottbus,
Konrad-Wachsmann-Allee 17, 03046 Cottbus,
Germany; +49-355-69-2815; e-mail leyens@tu-cottbus.de.
|