LATEST ISSUE |
||||
TMS QUICK LINKS: |
• TECHNICAL QUESTIONS • NEWS ROOM • ABOUT TMS • SITE MAP • CONTACT US |
JOM QUICK LINKS: |
• COVER GALLERY • CLASSIFIED ADS • SUBJECT INDEXES • AUTHORS KIT • ADVERTISE |
|
| Overview: Feature | Vol. 58, No.7, pp. 23-33 |
Biological Issues in Materials Science and Engineering:
Interdisciplinarity and the Bio-Materials Paradigm
L.E. Murr
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Questions? Contact jom@tms.org. © 2006 The Minerals, Metals & Materials Society |
|
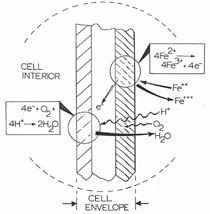
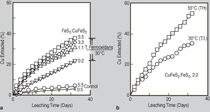
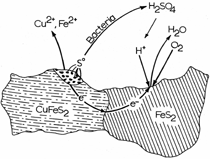
INTRODUCTION In 1969, the National Colloquy on the Field of Materials was held on the campus of Pennsylvania State University to assess and examine the emergence of materials science and engineering as a “new synthesis of disciplines.”1 After only a decade of development, materials science and engineering, while still in some degree of disciplinary uncertainty, was emerging as a model for interdisciplinarity and multidisciplinarity which prominently linked to various engineering disciplines, chemistry, physics, and mathematics. Its continued evolution along the lines of emergence of biophysics, combining biology and physics, or geochemistry, combining geology and chemistry, was questioned, but neither biomaterials nor bio-materials (the spectrum of biological sciences and materials sciences interdisciplinarity) was a prominent issue. By the end of the 1980s, a further assessment of the progress of materials science and engineering was made in the context of an effort “to conduct a comprehensive materials research and technology assessment for the next decade.” The result was a publication titled Materials Science and Engineering for the 1990’s: Maintaining Competitiveness in the Age of Materials.2 Here, inherently interdisciplinary areas included biomaterials prominently, but the true interdisciplinary issues of bio-materials education and research were not prominent, despite the fact that the synergistic elements which now defined materials science and engineering—structure, properties, synthesis/processing, and performance of materials—could find clear examples of bio-materials: prostheses, artificial organs, biosensors, drug delivery, wound management, and a plethora of medical equipment and devices. In addition, bioleaching and related biomaterials processing and processes were emerging along with biomimetics applied to materials-related innovations.
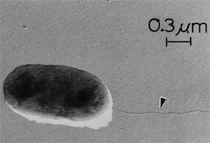
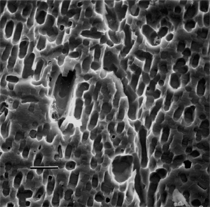
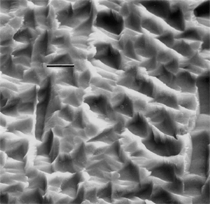
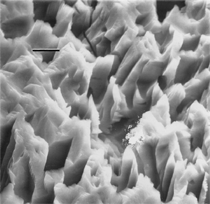
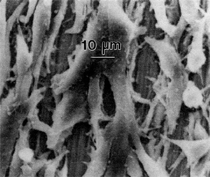
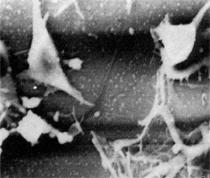
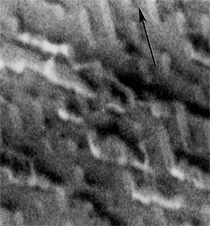
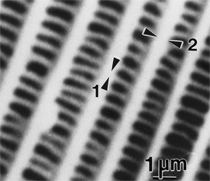
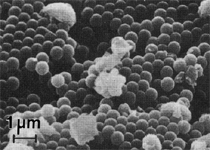
By the end of the 1990s, nanotechnology and nanomaterials became the emerging science and engineering initiatives worldwide, and more recent evolution of these concepts has touted convergent new technologies: the synergistic combination of nanotechnology, biotechnology, information technology, and cognitive sciences.3 These convergences elicit additional and imperative interdisciplinarity involving public health and the environmental sciences and engineering, especially as these relate to the manufacture and proliferation of nanomaterials, and in particular nanoparticulate materials. Here the biological and especially bio-materials implications bode prominently. This paper will review a few research examples—or case histories—of biological issues and interdisciplinary applications in materials sciences and engineering. These examples span more than three decades, including novel biomimetic materials developments and the development of systematic if not systemic assays to evaluate the cytotoxic potential for emerging nanoparticulate materials applications. In addition to demonstrating the applications of fundamental biological phenomena in materials extraction, processing, and performance (as related, for example, to degradation [biodegradation] or corrosion), this article will illustrate the characterization of biological and biomaterials microstructures as these relate to properties, processes, and performance. These examples and applications involving biological materials sciences will provide the basis for a bio-materials paradigm and the emphasis of the biological sciences in materials science and engineering curricula. Copper Leaching: in Dumps, In-Situ, in Vats Figure 1b and d also shows for comparison a higher temperature (thermophilic) Sulfolobus-like microorganism capable of catalytic activity at temperatures as high as 80°C and at pH values from 1 to 6 in contrast to the T. ferrooxidans, which becomes nonviable above about 40°C.9 Sulfolobus acidocaldarius and other thermophilic microorganisms have been isolated from acidic hot springs10,11 and other extreme environments. Microorganisms found in a variety of extreme environments (for example, temperatures well below freezing to near the boiling point) have become known as “extremophiles.” The role of bacteria, especially the synergistic or symbiotic catalysis of those illustrated in Figure 1 for heap, dump, or in-situ leaching of copper, is shown in Equations 1, 2, and 3, respectively, for pyritic porphyry copper waste or deposits. (All equations are presented in the Equations table.) In the presence of bacteria, the elemental sulfur formed in Equation 3 can be efficiently converted to sulfuric acid as shown in Equation 4. The efficiency of bacterial catalysis in these reactions has been demonstrated generally in the work of Lacey and Lawson,12 who showed that the rate of the reaction (see Equation 5) is nearly one million times faster for Equation 6. The ferrous-to-ferric oxidation in Equation 6 can be expressed generally as Equation 7, and is illustrated in Figure 2 in the context of the structure-function role of the cell envelope in oxidation catalysis. That is, regardless of the microorganism or its range of viability (Figure 1), its structure-function regime remains constant. The catalytic role of bacteria, including temperature symbiosis for T. ferrooxidans to Sulfolobus-like bacteria, as well as electrochemical cell effects involving the galvanic interaction in the leaching of metal sulfide systems, is illustrated on comparing the data in Figure 3. The metal sulfide particle mixtures (in Figure 3a) involved different size fractions of FeS2 and CuFeS2, producing variations in shake-flask pulp densities (solids/volume). The effects of bacterial catalysis, galvanic conversion, and the combined effects of galvanic conversion and bacterial leaching with both T. ferrooxidans (at 30°C) and the thermophilic Sulfolobus-like microorganism (at 55°C) are illustrated in Figure 3. Figure 4 illustrates schematically the conversion of chalcopyrite and the passivation of contacting pyrite where the anodic member (CuFeS2) having the lower rest potential (~0.52V) can react vigorously relative to the cathodic FeS2 (where the rest potential is ~0.63V). Similar results have been observed for large, bulk contacting systems as well, and for a variety of galvanic couples including FeS2, CuFeS2, and ZnS.9,13–15 The galvanic interaction between FeS2 and CuFeS2 (Figures 3 and 4) in acid medium can be described as follows. The interaction on the cathodic pyrite surfaces is shown in Equation 8, and on the anodic chalcopyrite surfaces in Equation 9. The overall, uncatalyzed reaction is observed to be that shown in Equation 10. The elemental sulfur formed in Equation 10 can be aggressively converted to sulfuric acid by bacterial catalysis as shown previously in Equation 4. Microbial-Influenced Corrosion and Materials BiodegradationElectrochemical attack involving electron flow through characteristic anodic and cathodic cells on a metal surface is regarded as the most general mechanism driving corrosion (i.e., an oxidation-reduction half-cell reaction). Equations 8 and 9 are specific examples. For general metals corrosion, metals (M) more reactive to hydrogen (referenced against the standard hydrogen electrode in the electromotive series) are assigned negative potentials and are considered to be anodic to hydrogen, as shown in Equation 11. For positive potentials, the reaction in Equation 11 is reversed. For a metal sulfide, the anodic reaction is similar, as shown in Equation 12, where n = 2. Figure 5 illustrates the resulting corrosion for an exposed (100) single-crystal pyrite surface in non-agitated sulfuric acid-ferric sulfate solution.16 In Figure 5a and b, corresponding to three-weeks and six-weeks exposure, the surface attack or corrosion is considerably less than that for the same lixiviant with the Sulfolobus-like microorganism added. Despite the fact that there is a reaction rate difference for the temperature difference (30°C versus 55°C), there is a clear indication of microbial influence, and of course the overall leaching comparisons in Figure 3 support this conclusion.15
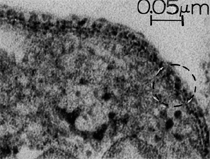
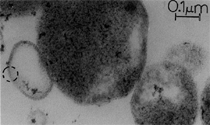
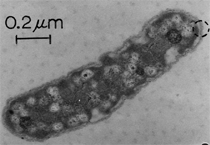
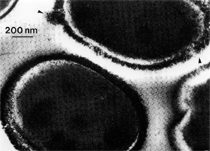
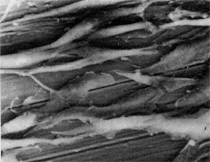
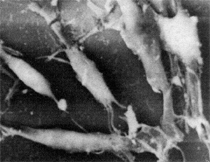
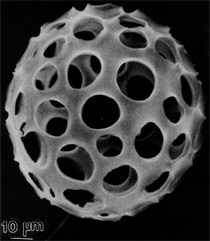
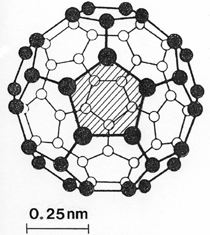
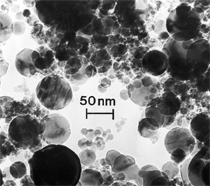
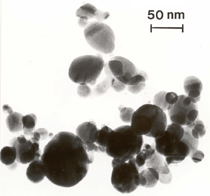
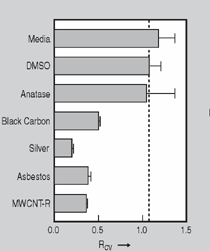
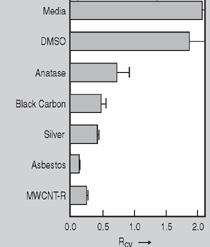
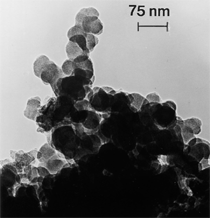
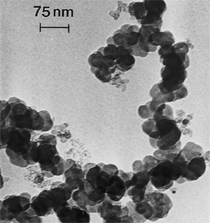
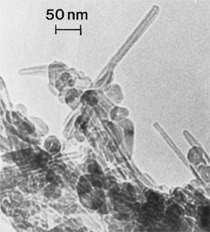
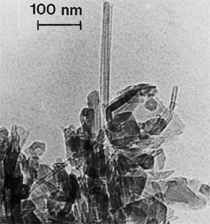
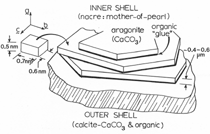
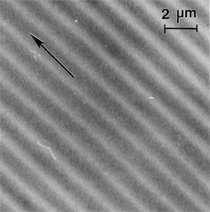
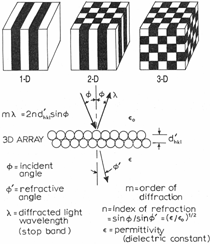
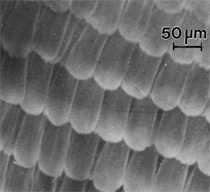
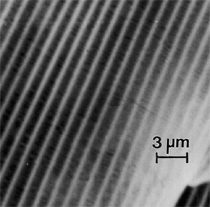
Anaerobic sulfate-reducing bacteria are also important in steel corrosion, especially associated with underground storage tanks and exposed pipes. Other metals are also similarly affected.17,18 Many steel structures as well as other engineering structures are degraded by bacteria and other microorganisms.19 Bacteria can form minerals by passive growth and as a result of metabolic activity. In addition, mineral degradation as a result of various mechanisms of biodegradation can occur. For example, heterotrophic fungi such as Penicillium (P.) simplicissimum are able to dissolve metals from various rocks by growing on sugar-containing substrates,20 which allows them to release citric acid, among other organic acids. The acids react with the mineral matter, dissolving metals through a chelating action. Aluminum dissolution in complex alumino silicates has been studied21 using P. Simplicissimum isolated from weathering basalt rocks.22 A recent publication by Benzerara et al.23 has described nanoscale environments associated with bioweathering of rocks. Biosorption and Bioremediation Figure 6a illustrates the accumulation of nanofibrils of uranium in a 0.2 μm surface cell envelope region of Saccharomyces (S.) cerevisiae.27 The uranium biosorbed on the cell envelope region in Figure 6a could be removed chemically, and the cells reused as a biosorbent.27 Other microbial cells take uranium into the cell body.27 In the case of uranium accumulation, Horikoshiet al.28 demonstrated that the abilities of microorgansims to accumulate uranium were as follows: actinomycetes > bacteria > yeasts > fungi. Figure 6b illustrates a more contemporary example of metal biosorption in living plants.30 The left portion of Figure 6b shows a low-magnification transmission-electron microscope (TEM) image of an alfalfa shoot embedded in a resin and microformed to a ~50 nm slice. The arrows indicate gold nanoparticles formed by the transport of gold atoms from the roots to the shoots. The high resolution TEM image to the right in Figure 6b illustrates these nanoparticles to be polyhedral crystals characteristic of metal quantum dots. Figure 6b also represents an example of using plants to extract gold or other heavy metals (such as uranium) from contaminated soil areas, a phenomenon referred to as phytoremediation.31 Silver uptake in alfalfa plants similar to gold in Figure 6b has also been demonstrated and these kinds of plant uptake features have been touted as a possible innovative process for synthesizing quantum dots.29 Oat biomass (or dead plant tissue) has also been demonstrated to possess the capacity to recover Au3+ ions from aqueous solutions forming gold nanoparticles as in Figure 6b.29 In fact, the size of the gold nanoparticles could be controlled by changing the pH.32 A range of agricultural byproducts, or dead biomass materials, have been demonstrated to have high affinities for heavy metal ions. These materials include waste wool33 and tree barks,34 among others. Biomaterials are among the earliest materials developments. For example, silver-tin amalgams as filling materials for teeth were first used by Chinese physicians around 700 AD.35 A dental amalgam containing bismuth, lead, and tin was introduced in France around 1826 while the contemporary Ag, Sn, Cu, and Hg amalgams evolved from the mid-1800s, especially in the United States.36 Although mercury has caused some concerns, dental amalgams continue to utilize roughly 40% mercury added to a particulate mix of ~40% Ag, ~28% Sn, ~30% Cu, and ~2% Zn.3,37 While other dental restorative materials have been developed,35 it is generally concluded that mercury-based amalgams will continue to be the restorative material of choice when aesthetic concerns are not important.38 Early development work and applications of titanium and titanium alloys for a variety of orthopedic and surgical reconstructive implants evolved from military and commercial efforts, especially in aerospace materials research. The high corrosion resistance and strength-to-weight ratio properties of Ti-6Al-4V in particular, along with high fatigue strength and nonthrombogenic behavior have made it a biomaterial of choice for several decades. However, recent concerns of titanium ion release as a biocompatibility issue, especially for long-term implants, have prompted searches for better biomaterials.39,40 Biocompatibility studies on titanium and Ti-6Al-4V have also been concerned with tissue (and cell) adhesion, and especially the performance of rough implant surface structure in contrast to very smooth implant surface structure. Figure 7 shows a simple comparison of rough versus smooth surface adhesion, or attachment, for fibroblast cells attaching to titanium (Figure 7a and b) and Ti-6Al-4V alloy (Figure 7c and d) in culture. There is a noticeable advantage for the rough surface for both materials in Figure 7. Over the past few years (especially since 2000) the field of biomaterials has grown rapidly. Advanced materials for diagnosis, drug release, and scaffolds for tissue engineering are currently being developed.42 The design and synthesis of artificial bone-like materials has been a recent outcome of this research.43 In recent years the efficiency of aerosol drug delivery, especially in nasal respiratory sprays from metered inhaler devices, has been enhanced by utilizing inert microcages, some inspired by zeolites and other confining structures.44,45 Tailored, cage-like silica structures consisting of micrometer-size particles with nanopores have been utilized to transport and control the release of drugs. Cyclodextrins, dendrimens, and other protein structures consisting of nanoscale matrices that form protective cages around delicate therapeutic molecules have also been developed for drug delivery.46,47 So-called “Trojan horse” proteins “smuggle” therapeutic molecules into cells using iron storage proteins like ferritin (a self-assembled protein cage-like structure) or other magnetic, iron-coated nanoparticles forming a bioferrofluid for magnetic drug delivery. These have been partly inspired by single-domain magnetic materials synthesized by magnetotactic bacteria which contain intracellularly produced crystals of magnetite (Fe3O4) or greigite (Fe3S4).48,49 Magnetic materials synthesis based upon magnetotactic bacteria and studies relating to bioinspired materials development represent a wide range of biomimetic approaches to materials chemistry, and materials sciences and engineering.50–52 Silica structures, especially cage-like silica structures, represent tailored or tunable size regimes which are frequently derived from the natural, biological world. Figure 8 illustrates a relatively common radiolarion, an ellipsoidal or spherical-shelled spumellaria; a marine plankton with a siliceous (opaline/polycystine) solid skeleton. Radiolaria are protozoa originating ~600 million years ago.53 Figure 8b shows, in contrast to Figure 8a, a C60 cage with pores or openings roughly 105 smaller than the radiolarian. The implication that nanocages such as the C60 molecule in Figure 8b can act as a therapeutic drug or other agent transport system must be tempered by potential adverse effects. For example, C60 has been described as a potential generator of singlet oxygen which is known to be a highly cytotoxic agent.54 In addition, Oberdörster55 has recently demonstrated that C60 accumulates as a toxic agent in fish brains, throwing some caution to the concept of drug transport in nanocage materials. Particulate materials, especially nanoparticle colloids, can have direct biomedical effects. Silver and its compounds are particular examples with known antibacterial effects in antiquity. Water stored in silver urns was known not to support pathogens while colloidal silver in water and other gels or salves has had numerous health benefits for hundreds of years.56 Recent applications of nanoparticulate silver have included open wound and burn treatment, and preliminary studies have shown that a 20 ppm silver colloidal suspension (~30 nm diameter) in purified water has a 100% cure rate for malaria.57 Titanium dioxide, especially as nanoparticulate anatase, is also an interesting antibacterial, with notable photocatalytic behavior. But ultrafine anatase has also been identified as cytotoxic, and in-vivo studies have shown that it can be severely toxic in the respiratory system.58,59 Figure 9a and b shows the appearance of commercially available TiO2 (anatase) and silver nanoparticulates, respectively, observed in the TEM. Figure 9c and d illustrates the cytotoxic response for anatase and silver in comparison to black carbon (BC), chrysotile asbestos nanofibers, and a commercial multiwall carbon nanotube aggregate material (MWCNT-R) for a murine macrophage cell line (Figure 9c) and a human macrophage cell line (Figure 9d), respectively. Figure 10a and b shows for comparison soot collected in a home with natural gas appliances, and the commercial BC represented in Figure 9c and d, respectively. Figure 10c and d shows for comparison MWCNT aggregates in a kitchen using natural gas to cook and MWCNT-R represented in Figure 9c and d, respectively. The black carbon in Figure 10b and the MWCNT material in Figure 10d might be considered to represent surrogate materials for the anthropogenic samples shown in Figure 10a and b. These might be considered to be cytotoxic in the context of the results shown in Figure 9c and d.60 The use of chrysotile asbestos in Figure 9c and d as a positive control exhibiting a cytotoxic response may serve as a special caution for nanoparticulate materials development and their nanotechnology applications. Chrysotile asbestos is probably the oldest and most versatile nanofibrous material, having achieved nearly 4,000 different product applications or uses from circa 500 B.C. to the present time.61 But it was demonstrated to be a prominent cancer cause in the late 20th century, after having been described as a major health problem as early as 70 A.D. by Pliny the Elder. Consequently, the emerging prospects for nanobiotechnology and even broader nanotechnology and nanomanufacturing must recognize the complex interactions of nanoparticulates in biological systems. Biomimetics is often considered the abstraction of good designs from nature. As they apply to materials innovation and development, biological systems can exhibit wide arrays of multifunctional materials. Around 1940, Swiss engineer George deMestral returned from a hike with his dog and noticed both he and the dog were covered with burrs. Upon examining the burrs under a microscope, deMestral was struck with the idea of a fabric fastener. Velcro®, the quintessential biomimetic design example, was born. In a more recent example, mollusk shells, particularly abalone, have been recognized as hierarchical, structural composites optimized for toughness, despite the fact that they are composed of relatively weak calcium carbonate plates bound together by an organic glue, or mortar. For example, the tensile strength and toughness of the CaCO3 (aragonite) plates (Figure 11) are roughly 30 MPa and < 1 MPa-m1/2, respectively, while the tensile strength and toughness of the inner shell (or mother-of-pearl of the abalone) varies between 100 MPa and 300 MPa, and corresponding fracture toughness between 3 MPa-m1/2and 7 MPa-m1/2.62–64 As illustrated in Figure 11, while the inner (nacre) portion of the abalone shell is composed of ~86% aragonite blocks joined by ~10% conchiolin or complex fossil protein (sclero protein) and water, the outer portion of the shell is composed of calcite (CaCO3) with an increasing amount of conchiolin near the outer surface. This laminate microstructure of aragonite has inspired a new class of structural materials called metallic-intermetallic laminate composites.65 A Ti-Al3Ti laminate has demonstrated impressive ballistic performance as a consequence of high strength and fracture toughness, and thermal management attributes.65 Of course in the abalone, the multifunctional attributes not only include these unusual mechanical properties, but also a self healing and translucent, lustrous, colored pearl material which can be used as the basis for cultured pearls. The color of mother-of-pearl is the result of diffraction from the one dimensional aragonite lattice illustrated schematically in Figure 11, and shown in the scanning-electron microscopy (SEM) view of a fractured nacre section in Figure 12a and b. The resulting blue-to-orange color corresponds to spacings from roughly 400 nm to 600 nm, respectively (Figure 11). These structural color effects are also illustrated by tilting a compact disc (CD) in the light, which alters the effective track spacings and the incident angles to diffract all the colors included in the optical portion of the spectrum (~350 nm to 800 nm wavelengths). Figure 12c and d shows SEM views for a clean CD and a CD with digital information written onto it, respectively. The arrows in Figure 12c and d provide a directional reference for the grooves or tracks which are interrupted in Figure 12d to produce the digital tracking or data storage. Color in biology is due to a variety of optical phenomena including interference, diffraction, absorption, refraction, reflection (or scattering), and diffusion. Diffraction effects, as noted previously, can occur by reflection from finely ridged surfaces (Figure 12c) acting as a diffraction grating. Periodic microstructures on the scale of color wavelengths create this optical diffraction grating. Structural coloration in nature has inspired innovations in nano-bio-optics, nano-optics, or photonics.66–69 Photonic crystals or spatially periodic structures fabricated from dielectric materials have different retractive indices. These materials were first proposed by Yablonovich70,71 and John72 as optical band gap structures. Photonic crystals73 or so-called superatom lattices can be one-, two-, or three-dimensional (1-D, 2-D, or 3-D) structures, illustrated schematically in Figure 13a. These structures can be patterned from two different dielectric materials or interconnected air spaces or porous materials. These arrays, especially 3-D arrays, can produce structural color by diffraction as illustrated schematically in Figure 13b. Figure 14 illustrates some common examples of biological or natural structural color regimes. Figure 14a to c illustrates yellow-orange butterfly wing scales and color-generating scale microstructures. Figure 14d shows a typical opal structure, which represents a natural face-centered cubic SiO2 sphere structure or 3-D photonic crystal structure. As the silica (SiO2) sphere size varies, the diffracted color will correspondingly vary. In addition to the structural color generation illustrated in Figures 12 and 14, there is the pigmentation coloring by organic dyes and particulate pigments, both microscopic and nanoscopic. Fiber reactive dyes are formed inside fibers and produce light and wash “fast” colors, while dyes such as indigo can become trapped in nanoporous fibers such as palygorskite to produce durable color systems such as Maya blue.74,75 Indigo is a plant-derived pigment produced in anaerobic batch processing with Clostidium bacteria. Common examples of particulate coloration due to optical absorption occur in glass where nanoparticles and nanoclusters produce a wide range of colors: Co (blue), Mn (amethyst), Se (red), Ti (yellow), Au (~10 nm–ruby; < 10 nm–cranberry), and Fe (green).76 Many biological (natural) regimes exhibit both structural and pigmentation coloring. Peacock feathers are a good example. The blue peacock feather “eye” results by structural coloring while the associated red and black coloring is due to pigmentation.68 Beetles also exhibit structural colors,67 but a recent discovery in Queensland, Australia, of a green-colored weevil was the first observation of an opal color structure in animals. The close-packed opal lattice on this beetle was found to consist of hemispherical scales.77,78 Around 2000, biologist Kellar Autumn at Lewis and Clark College in Oregon was fascinated by the ability of a gecko to navigate ceilings and walls with ease. Together with an undergraduate research student, Wendy Hansen, it was discovered that the gecko has closely packed foot hairs called setae (~100 µm in length) with pads on their ends called spatulae (~200 nm in diameter). These hairs create a van der Waals force of ~200 μN and there are roughly 15,000 setae/nm2 on the gecko foot. Not only do geckos attach by what is tantamount to a “dry” adhesive effect, but the hairs were discovered to be self-cleaning, since the gecko can attach to dirty surfaces.79 Consequently, a self-cleaning, dry adhesive material was under development. Yurdumakan et al.80 have described synthetic gecko foot hairs configured from aligned growth of multiwall carbon nanotubes similar to those illustrated in Figure 10c and d. One centimeter square of this synthetic adhesive can support ~1 kg by the combined van der Waals force created. Bio-materials as a paradigm outlined in this article involves bioleaching and biomineralization, biologically or microbial-assisted corrosion, biosorption and bioremediation, bio-nano-photonics and photonic materials, nano-bio-materials, functional bio-materials, including multicomposite bio-materials, biomimetic materials, and biologically inspired materials innovations. Since materials in a broad sense imply materials science and engineering, bio-materials can be considered to connect the broad discipline of biology to an existing multidisciplinary area. Interdisciplinarity or interdisciplinary studies in the context of research implicit in the examples provided herein demonstrate a process or processes to address broad and complex problems not adequately dealt with by a single discipline or profession.81,82 Biophysics, biochemistry, biomolecular nanotechnology, etc., integrated with materials science and engineering provide an even broader context to bio-materials including cross disciplinarity and transdisciplinarity, in addition to interdisciplinarity and multidisciplinarity.82 Biophysics, biochemistry, and bioengineering represent biological issues in traditional disciplines of physics, chemistry, and engineering, respectively, while bio-materials integrates biology with materials science and engineering—in a broad sense, not just biomaterials as a subset of materials types. Materials engineering, or more broadly materials science and engineering curricula, need to re-think the role of chemistry and physics and the necessity to introduce biology and the biological sciences as a part of the basic sciences core in order to embrace the development of the biomaterials paradigm as a reconfiguration of the materials science and engineering curriculum. Bio-materials in the context of engineering interdisciplinarity must of necessity become more pervasive in both undergraduate and graduate materials curricula in support of increasing emphasis in bio-materials research and developments worldwide. Biological structures, properties, processes, and their associated performance mimic the basic tenets of materials science and engineering. Life constitutes the ultimate materials, the construct of living matter. Living cells and their constituents are the manifestation of multi-functional, self-organizing (or self-assembling), nano-micro-mesocomponents, the end point and starting point (alpha and omega) for biomimetics. Consequently bio-materials is a necessary, paradigmatic inclusion in contemporary materials science and engineering education. Research incorporated as examples in this paper has been supported in part by several projects funded by the Southwest Consortium for Environmental Research and Policy and a Mr. and Mrs. MacIntosh Murchison Endowed Chair. The help of Micah Baquera, Erika Esquivel, and others in creating selected SEM images utilized in this paper is especially acknowledged. References L.E. Murr is a professor in the Department of Metallurgical and Materials Engineering at The University of Texas at El Paso. For more information, contact L.E. Murr, The University of Texas at El Paso, Department of Metallurgical and Materials Engineering, 500 West University Avenue, El Paso, TX 79968, USA; (915) 747-6929; fax (915) 747-8036; e-mail lemurr@utep.edu. |
|||||||||||||||||||||||||||||||||||