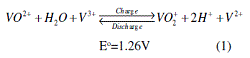|
Environmental concerns about using
fossil fuels, and their resource
constraints along with energy security
concerns, have spurred great interest
in generating electrical energy from
renewable sources. The variable and
stochastic nature of renewable sources,
however, makes solar and wind power
difficult to manage, especially at high
levels of penetration. Electrical energy
storage (EES) is necessary to effectively
use intermittent renewable energy, enable
its delivery, and improve the reliability,
stability, and efficiency of the
electrical grid. While EES has gained
wide attention for hybrid and electrical
vehicle needs, public awareness and
understanding of the critical challenges
in energy storage for renewable integration
and the future grid is relatively
lacking. This paper examines the benefits and challenges of EES, in particular
electrochemical storage or battery technologies,
and discusses the fundamental
principles, economics, and feasibility of
the storage technologies.
| HOW WOULD YOU... |
…describe the overall significance
of this paper?
With increasing use of renewable
power generated from intermittent
sources such as solar and wind,
interest has grown in research and
development of stationary electrical
storage. To help the materials
community gain insight into the
emerging area, this paper offers an
overview on the needs, requirements,
and potential technologies.
…describe this work to a
materials science and engineering
professional with no experience in
your technical specialty?
A number of existing and emerging
technologies are potential candidates
for energy storage applications. All
these technologies are, however,
facing challenges to meet economic
and performance targets for wide
market penetration, which requires
substantial advances in materials,
design, system engineering, etc.
…describe this work to a
layperson?
Batteries are widely used to store
electrical energy for electronics
and now hybrid vehicles. Can these
batteries be used to store renewable
energy? The answer may be yes. But
they have to be capable of storing
it at large scales and being costeffective.
Substantial advancement
is required for the current battery
technologies, along with nonelectrochemical
means, to meet the
economic and performance targets. |
INTRODUCTION
Current annual worldwide energy
consumption is estimated to be 15 TW
(1 TW = 1012 watts).1 Approximately
80% of today’s energy is supplied from
fossil fuels: oil (34%), coal (25%), and
natural gas (21%).2 Biomass is 8% of
the energy supply, nuclear energy 6.5%,
hydropower 2%, and other technologies
such as wind and solar make up the rest.
Even with aggressive conservation and development of new, higher-efficiency
technologies, worldwide energy demand
is predicted to double to 30 TW
by 2050 and triple to 46 TW by the
end of the century. Electricity will not
only continue to be the dominant player
(40% of all energy consumption in the
United States in 2002), but its share will
increase at a faster pace than overall energy
consumption.3 At the same time, oil
and natural gas production is predicted
to peak over the next few decades. Coal
production accounts for about 40% of
the electricity generated in the world;4
abundant coal reserves may maintain
current consumption levels longer than
oil and gas. However, every kWh of
electricity generated by burning coal coproduces
an average 1,000 g/kWh lifecycle
CO2 emission, a greenhouse gas
that is widely considered as the primary
contributor to global warming.5,6 In the
United States, coal power plants emit
1.5 billion tons of CO2 per year while
emissions from developing countries
are accelerating. While emitting fewer
greenhouse gases, burning oil and natural
gas results in a lifecycle CO2 emission
of 800 g/kWh and 400–500 g/kWh,
respectively. To reduce greenhouse gas
emissions, many countries are adopting
tough regulations (i.e., cap-and-trade
or variants) and carbon trading, which
benefits industries with a small carbon
footprint and requires those producing
higher emissions to purchase carbon allowances.
The environmental concerns about
fossil fuels and their constraints, combined
with energy security concerns,
have spurred great interest in generating
electrical energy from renewable sources.
Solar and wind energy are among the
most abundant and potentially readily
available.5,7,8 The solar radiation energy
the earth receives in one hour is enough
to meet worldwide energy requirements
for a year. Capturing a small percentage
of potential wind energy could also
contribute significantly to meeting the
world’s electrical energy requirements.
While advancements in technology are
still needed to harvest renewable energy
economically, solar and wind power
technologies have grown quickly. Globally,
total installed wind power reached 74.3 GW in 2006 and 94 GW in 2007.9
The World Energy Council estimates
that new wind capacity worldwide will
total up to 474 GW by 2020. The output
from photovoltaic (PV) module installations
increased to 2.5 GW by 2006 and
is currently growing at 40% worldwide.7
More countries and states are pushing
for aggressive portfolios of renewable
energy. In Denmark, penetration of
wind power has already reached 17% of
total power generation, and renewable
generation will be doubled by 2015. In
the United States, Hawaii has 20% of
its electricity generated from wind and
is gearing up for 70% to be generated
from renewable sources by 2030. California
is targeting 20% to be generated
from non-hydro renewable sources by
2010 and no less than 33% by 2020. If
hydro power is considered, California is
at ~25% currently. The U.S. government
has called for doubling the production
of alternative energy in the next three
years. Renewable energy is a centerpiece
of the president’s new economic
plan to improve the U.S. economy.
However, solar and wind are not
constant and reliable sources of power.
The variable nature of these renewable
sources makes any discussion of
reliable instantaneous levels of solar
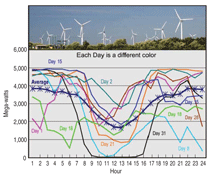
or wind power moot. For example, as
shown in Figure 1, wind power profiles
in Tehachapi, California vary over minutes,
hours, and days, while peaking at
night when demand is low. During the
day, wind power can be a few GW in
some moments and only a few MW
and even zero in others. Similarly, solar
power is generated only in the daytime
and varies when clouds pass by. Storage
of electrical energy has been recommended
as a way to eliminate this variability
and adapt to the demand. Some
view electrical energy storage (EES) as
the “Achilles Heel of Renewable Energy.”10 Meanwhile, there has been intense
discussion among the scientific community,
renewable energy developers, and
utility industries on the implementation
and economic benefits of EES. Different
scenarios are being considered. The
most optimistic view is that renewable
energy could be balanced out when integrated
into a large electrical grid. The
fast growth in renewable energy and aggressive
renewable portfolio standards
being set worldwide requires answering
some fundamental questions: Can
significant penetration of renewable energy
be implemented without storage?
If storage is indeed needed, what is the
status of storage technologies relative to
technical and cost targets; consequently,
what research and development are
needed to advance these technologies?
In this paper, we attempt to answer
these questions by discussing the need
for electricity storage, examining the
economic and technical status of potential
storage technologies, and exploring
fruitful directions for research and development.
| TECHNICAL AND ECONOMIC CONSIDERATIONS OF EES |
Performance requirements of EES for stationary use depend on
the application markets. For example, to regulate frequency, energy
storage capacity may not need to be long-lasting—seconds or minutes
are sufficient—but it must have a long cycle life because the
system is likely to encounter multiple daily discharge events. High
charge and discharge rates or high current densities are important
although the state-of-charge of the storage system typically will not
move over a wide range. In comparison, load shifting requires systems
of up to MWh or even GWh levels that are capable of a high
ratio of energy storage capacity to discharge power rating so that discharging
can occur at a designated power for relatively longer periods,
typically a few hours or more. For this type of application, high
round-trip energy efficiency and a long deep-cycle life, along with
low operation and maintenance costs are principal drivers. While
storage for renewable power may cover the whole spectrum of discharging
times, many agree this type of application
demands technologies or systems that may
range from a few kWh to MWh, and importantly,
are capable of a long duration (hours) of storage.
Unlike vehicle applications that have constraints
on weight and volume, high-energy densities may
not be strictly required for stationary applications.
Also, the grid and renewable applications often
require a quick response from the storage that can
reach full power in a matter of a second. Finally,
as utility assets, EES must have a long lifetime
(e.g., >15 years).
Reliability/Safety of energy storage systems
must be addressed for applications at scale due
to the amount of stored energy. Many electric energy
storage technologies, especially those that
operate electrochemically, have the potential to
release their energy rapidly if the structure fails or
certain temperature limits are exceeded. The uncontrolled
energy release can range from a thermal
runaway event that simply drains the storage
system of its energy to an explosive discharge of
energy. Better safety and reliability requires the
use of inherently safe materials/chemicals and
better engineering of the storage systems against
rapid, explosive releases of energy.
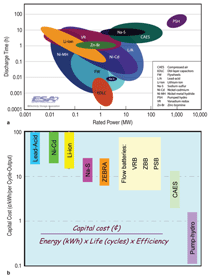
Cost is probably the most important and fundamental
issue of energy storage, which can be
expressed both in terms of the unit cost of power
($/kW) and the unit cost of energy capacity
($/kWh) or the per-cycle cost (¢/kWh). Unit cost
of energy capacity better measures the capital cost
for power management, while $/kW or ¢/kWh
makes sense more for energy arbitrage. Different
applications have different cost tolerances. Take
load shifting and stationary renewable storage as
an example. In the author’s opinion, the cost of
electricity storage probably needs to be comparable
to the cost of generating electricity, such
as from natural gas turbines at a cost as low as
4~5 ¢/kWh per cycle. Thus, to be competitive, the
capital cost of storage technologies for energy applications
should be comparable to or lower than $180~225/kWh,
assuming a lifecycle of 15 years or 4,500 cycles (25 cycles per
month). A capital cost of $800~1,100/kWh or less is desired if the
technology can last 5 hours at the name-tag power. Unfortunately,
most current technologies, except probably for pump-hydro (Figure
A), cannot meet the economic requirement even without accounting
for carrying charges, operation/maintenance (O&M), and
replacement costs. There can be some room for renewable power
generated at a higher cost than traditional natural gas turbines; but
low cost is the primary driver for broad market penetration of storage
technologies. Technologies capable of serving multiple markets,
such as for both regulation and load-shifting, can be more
competitive and are thus preferable to those serving a single function.
Ultimately, cost reduction will rely on technology advancements
to improve reliability, cycle life, efficiency, use of less expensive materials, etc.
|
THE NEED
FOR ENERGY STORAGE
IN THE FUTURE GRID
Energy storage is an established,
valuable approach for improving the
reliability and efficiency of electricity
transmission and distribution (T&D).
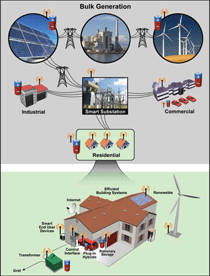
Sited at various T&D stages (Figure 2),
storage can be employed to regulate frequency,
control power quality, serve as
reserve power, and provide load leveling
or shifting.
Frequency is regulated using EES to
balance generation and demand, which
can fluctuate from second to second and
minute to minute, to prevent disruptions
that cost up to tens of billions of dollars
annually in the United States alone.
Electrical energy storage can also serve
as reserve power to improve grid stability,
for example, to avoid voltage collapse
and a cascading outage or a blackout
such as that which occurred August 11,
2003 in eastern Canada and the United
States. Reserve power is important for
internet and communication centers,
which currently account for over 1.5%
of the total utility power consumption in
the United States, according to a report
by Lawrence Berkeley National Laboratory.
These digital communication
centers are very sensitive to electricity
fluctuation and disruptions. The most
common applications of EES include
improving the economics of power supply
by load leveling or shifting, which
involves storing electrical energy at one
time and releasing it at another. Preferably,
the energy is stored when excess is
generated and released at times of greater
demand. Load leveling is one kind of
load shifting in which energy is stored
when it can be produced cheaply (at offpeak
times, for example) and released at
peak times when it is more valuable.
To date, however, only 2.5% of the total
electric power delivered in the United
States passes through energy storage.
That amount is primarily limited
to applications associated with pumped
hydroelectric storage. The percentages
are higher in Europe and Japan, at 10%
and 15%, respectively, largely because
of favorable economics and government
policies.11
Generally, existing grids that traditionally
rely on base loads and are
backed up by fossil-burning peak plants
and spinning reserves, may handle some
level of renewable. While their operating
conditions may be acceptable, current
grids face great challenges to reach
significant levels of penetration with intermittent
renewables and meet evolving
changes in demand. However, there appears
to be a critical limit over which the
grid essentially becomes unreliable. The
limits in percentage of renewable penetration
reported so far range from 10%
to over 30%, generally falling between
15 and 20%, depending on the size of
the grid, renewable profiles, etc.10 Reported
by the Electric Power Research
Institute, California, with penetration
of wind power approaching 20%, the
grid in Hawaii has already experienced
instability.10 One may argue that Denmark
handles a 17% penetration of wind
power without much use of storage. According
to Incoteco, a Denmark-based
energy consulting company, Denmark
uses only 8–9% of the wind power generated.
The rest is exported into Germany,
Norway, and Sweden, which act
as giant energy sponges with the help of
some installed storage capacity. In anticipation
of building another 4.5 GW
offshore wind farm, Denmark is actively
studying and evaluating suitable energy
storage options.
An even greater challenge is that renewable
sources are often localized and
abundant in remote areas. With a great
deal of wind or solar energy generation,
sudden shifts in the local wind patterns
or sunlight intensity can cause significant minute-to-minute imbalances
between generation and load, resulting
in changes in system frequency. A
large change can trigger an automatic
emergency shutdown of generation
and cause a blackout. For example, on February 27, 2008, a cold front moved
through west Texas and winds died in
the evening just as electricity demand
was peaking. Generation from wind
power in the region plummeted rapidly
from 1.7 GW to only 300 MW. The sudden
loss of wind power, and the lack of
alternative electricity supply, forced grid
operators to cut power to some offices
and factories for several hours to prevent
a statewide blackout. To ease the
bottleneck limiting the development of
wind resources in the state, it was proposed
to build new transmission lines
linking wind farms to customers. In addition
to the high cost ($1.2–6 million
per mile12), new transmission lines take
a long time to build and negatively impact
the environment by cutting through
once-natural landscapes. The concerns
over renewable transmission during the
economic downturn shelved a plan to
build the 4 GW Pampa Wind Form. Alternatively,
integrating EES could help
manage the variability in renewable
electricity while reducing economic
and environmental impacts. One of the
important benefits of employing EES is
indeed to allow for increasing penetration
of renewables and deferring investment.
Storage technologies become increasingly
valuable with decentralized
generation resources and users, a trend
expected for the future “green” grid.13
Additionally, EES can be a valuable
approach for improving the economics
and utilization of renewable energy.
Electrical energy storage can be sourced
either at the generation site or close to
loads (see Figure 2). If installed near a
wind or PV farm, an EES system can
store/release energy via load shifting in
accordance with the generation profile.
For wind power, this is called “firming
and shaping” because it changes
the power profile of the wind to allow
greater control over dispatch. Similarly,
when using EES with PV generation the
extra electrical energy generated during
daytime is stored for use at night when
there is no power output. Load shifting
helps improve the economy of renewable
power and management of the balance
between supply and demand. If
pumped directly into electrical grids,
intermittent renewable power is likely
to disturb the balance between demand
and supply.
A further incentive to employ EES is that it can help reduce greenhouse gas
emissions and enable the utility industry
to meet anticipated carbon emissions
limits. Without storage, renewable penetration
would be backed up by fossilburning
turbines that run at low efficiencies
and release greenhouse gases, mitigating
the benefits of renewable energy.
Based on a study by Carnegie Mellon
University,14 fossil back-up would penalize
the reduction in CO2 emissions
from wind power by about 22% and
the corresponding NOx saving by 70%.
The U.S. Department of Energy (DOE)
is targeting 20% wind penetration by
2030 or integrating approximately 300
GW of wind energy into the U.S. grid.
Approximately 50 GW of peaking gas
turbines would be used to compensate
for the variability of the wind’s power
output.15 Replacing gas turbines with
electrical storage would greatly reduce
greenhouse gas emissions.
Along with increasing integration of
renewables, the future grid is expected
to be able to provide fuel (i.e., electricity)
to plug-in hybrid vehicles (PHEVs)
and allow two-way communication and
digital balancing of demand and supply
(i.e., a smart grid). If most PHEVs are
charged at night, wind energy that peaks
at night may have a positive impact on
the grids over time. However, adding
solar power would shift the overall generation
peak to daytime. The smart grid
will be driven by the desire to improve
capacity, which stands at about 40% in
the United States, by shifting the demand
curve through either incentives
or controls. Electrical energy storage
has been suggested as a key enabler for
the future grid (Figure 2). The end-user
storage system would store electricity
from renewable generation or transmission
line energy when it is cheap and
charge vehicles or send electricity back
to the grid during expensive peak hours.
In addition, storage offers an effective
alternative approach to help balance the
system as a means to adapt production
to demand while improving capacity.
Given these benefits, and that deployment
of EES may be more environmentally
acceptable and potentially less
detrimental to the economy and society
than other types of upgrades, the U.S.
Energy Independence and Security Act
of 2007 authorized the DOE to develop
and demonstrate storage technologies
for utility applications.16
In short, high penetration of renewable
energy and greater emphasis on
energy efficiency, along with more
electric vehicles and the use of smart
grid technologies, is creating an urgent
need for EES technologies that
can ensure optimal and efficient use of
generation, transmission, and distribution
resources in a carbon-constrained
world.
POTENTIAL
TECHNOLOGIES: STATUS AND CHALLENGES
A number of technologies can be
potential candidates and some of them
were already demonstrated for renewable
and utility applications. The storage
characteristics, along with cost, are
summarized in Figure A.17,18 Electrical
energy storage can be realized either by
direct storage in electrical charges or
by conversion of electrical energy into
other forms of energy that may include
chemical, potential, kinetic, etc. Direct
storage technologies, such as electrochemical
capacitors or supercapacitors,
are highly efficient (close to 100%), but
have low-energy density and discharge
in seconds or sub-seconds. Thus, these
technologies, along with storage in kinetic
energy (i.e., flywheel) are useful
for power management.
The electrical storage in potential
energy, such as pump-hydro and possibly
compressed air electrical storage
(CAES), can be attractive options for
bulk energy storage up to the GW level.
With the lowest cost per cycle among
the known technologies, a number of
pump-hydro storage plants have been
built and operated worldwide. Compressed
air electrical storage plants use
off-peak electricity to compress air into
an air storage system. When the grid
needs additional electrical power, air is
withdrawn from the store, heated, and
passed through an expansion turbine
driving an electrical generator. There
have been a few demonstrations including
the municipal utility CAES plant being
developed in Iowa. However, these
two types of storage require a large initial
investment, and more importantly,
have geological and environmental
limitations. Besides, the effectiveness
and economy of CAES has not yet been
fully proved, and the technology is not
truly clean, consuming about 35% of
the amount of premium fuel by a conventional
combustion turbine (CT) and
thus producing about 35% of the pollutants
per kWh generated from a CT.
The largest group of technologies
for stationary applications is probably
electrochemical storage or batteries that
can efficiently store electricity in chemicals
and reversibly release it according
to demand. Early technologies include
lead-acid batteries that store and release
electricity via a reversible electrochemical
conversion of lead to lead sulfate at
the anode and quadrivalent lead oxide
to lead sulfate at the cathode in a concentrated
sulfuric acid electrolyte. Over
the last hundred years, lead-acid battery
technology has been the most widely
used of any electrochemical storage
medium. These batteries have been applied
to virtually every area of industry,
and their sales constitute 40–45% of all
battery sales in the world.19 Since they
are readily available, lead-acid batteries
have been tested for utility applications,
particularly for power regulation, power
quality, and reliability control. The largest
installation is a 40 MWh system
built in 1988 in Chino, California that
was used for load leveling at the Chino
substation of the Southern California
Edison Company. However, lead-acid
batteries have a short cycle life and a
high per-cycle capital cost (Figure A),
in spite of the relative low energy cost
in dollars per kWh. Interestingly, progress
has been made lately by modifying
the traditional lead-acid battery by the
lead anode with active carbon, a material
used in supercapacitors. Axion
Power International, Inc. and East Penn
of Pennsylvania are actively developing
and demonstrating the technology for
electrical grid applications. Evaluation
at Sandia National Laboratory indicated
a much improved cycle life over the traditional
lead-acid batteries.
Other early technologies include
varied nickel batteries that all share the
same cathode (nickel oxyhydroxide in
the charged state). In an aqueous KOH
electrolyte, the cathode discharges to
form nickel hydroxide. The anodes are
either metals that oxidize to form a hydroxide
or metal hydrides that lose hydrogen
when discharged. Potential candidates
for utility applications include
nickel-cadmium, nickel-zinc, nickelhydrogen,
nickel-metal-hydride, etc.
Among the most notable chemistry for
utility applications are probably nickelcadmium
batteries.11 This battery chemistry
is characterized by a good energy
density and excellent power delivery
capability. A large system was commissioned
in 2003 in Fairbanks, Alaska,
to provide 27 MWac power for a short period of time (up to 15 minutes) until
back-uo generation comes online. The
battery system nevertheless uses toxic
cadmium, which is a serious environmental
hazard requiring special disposal.
Also, it is susceptible to overcharge,
and the direct-current-to-direct-current
efficiency is only about 60~70%. The
overall cost is still high (Figure A).
The high cost, technical concerns
about electrochemical performance, and
environmental hazards over the traditional
technologies have spurred efforts
to optimize technologies that were developed
in the past few decades for the
particular applications. These technologies
may include, but are not limited
to, redox flow batteries, sodium-oxide
membrane batteries, some unique lithium-
ion batteries, etc.
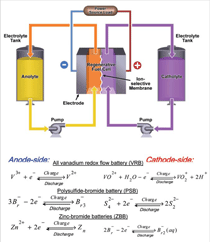
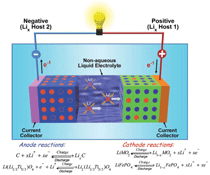
Flow Redox Batteries
Flow Redox Batteries (FRB) store
electrical energy in two soluble redox
fluids contained in external electrolyte
tanks sized in accordance with application
requirements (Figure 3). Aqueous
liquid electrolytes are pumped from
storage tanks to flow-through electrodes,
where chemical energy is converted to
electrical energy (discharge) or vice
versa (charge). Between the anode and
cathode compartments is a membrane
that selectively allows cross-transport of
non-active species (e.g., H+, Na+, etc.)
to maintain electrical neutrality and
electrolyte balance. Unlike traditional
batteries that store energy in electrodes,
FRB batteries are more like regenerative
fuel cells in which the chemical energy
in the incoming fuels is converted into
electricity at the electrodes. As such the
power and energy capacity of an FRB
system can be designed separately. The
power (kW) of the system is determined
by the size of the electrodes and the
number of cells in a stack, whereas the
energy storage capacity (kWh) is determined
by the concentration and volume
of the electrolyte. Both energy and
power can be easily adjusted for storage
from a few hours to days or even weeks,
depending on the application, which
is another important advantage for the
renewable integration. Generally, FRB
sustains no damage to the cells when
completely discharged, although overcharging
may need to be avoided. There
is only negligible self-discharge irreversible
loss in optimized flow systems,
and generally no problems associated
with short circuiting. The liquid electrolyte
and intimate interfaces with electrodes
make high current densities and
quick response (in a matter of sub-seconds)
possible for utility applications.
Simplicity in cell and stack structure
allows for building large systems based
on module design, which is another important
advantage for electrical grid applications.
The foundational work on FRB was
carried out at NASA in the early 1970s
for space applications.20,21 The early
NASA FRB employed iron and chromium
redox couples (Fe2+/Fe3+||Cr2+/Cr3+),
both acidified with hydrochloric acid,
giving an open circuit voltage of about
1.2 V. A critical issue with the early redox
system was the permeation of iron
species into the chromium electrolyte
and vice versa, causing quick performance
degradation. Interest in optimizing
the Fe-Cr RFBs for grid applications
has been renewed lately. To overcome
the cross-transport issue, Maria Skyllas-Kazacos of the University of New South
Wales, Australia, invented an all-vanadium
redox battery (VRB) in the mid
1980s.22,23 Unlike the chromium-iron
cell, VRBs use the same element, vanadium,
in the sulfuric-solution anolyte
and catholyte. The energy conversions
in the battery are realized via changes
in vanadium valence states through the
following electrode reactions:
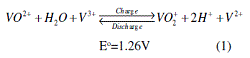
The overall electrochemical reaction
gives a standard cell voltage of 1.26 V
(at 1M and at 25°C). The chemical stability
of the sulfuric electrolytes, however,
limits the all-vanadium operation
to the 10–40°C range, with an energy
density about 25 Wh.kg–1.24
In RFB’s acidic environment, inert,
high-surface-area carbon or graphite-based
materials in forms such as felt or
porous structure were often bonded with
a conductive substrate such as a conductive
polymer as a current collector to
form electrodes.25–27 The fast kinetics
of the vanadium redox reactions allows
high columbic and voltaic efficiencies
often without the use of expensive catalysts.28 Perflorinated sulfonic acid membranes
(e.g., Nafion membranes) generally
have been used due to their high
ionic conductivity (0.07~0.23 Scm–1 for
H+) and good chemical stability in the
electrolytes.29–30 Recent studies attempted
to modify the Nafion membranes for
improved permeability and selectivity
as well as chemical compatibility with
V5+ electrolytes.31–33 Up to 92% cell efficiency and 80% from 10 KW have been
reported.34,35
The first large VRB (50 KW/200
kWh) was built by Kashima-Kita Electric
Power, a Mitsubishi subsidiary, and
went into operation in 1995. Since then
systems up to MWh levels were developed
and demonstrated. In 2005, Sumitomo
Electric Industries successfully
demonstrated a 4.0-MW/6.0-MWh system
at the 32-MW Tomamae wind farm
on Hokkaido in northern Japan. A cycle
life of >6,000 cycles (80% depth of discharge)
and a calendar life up to 8 years
(longer with replacement of components)
has been demonstrated for small
systems.21
In addition to the aforementioned, a
number of other flow battery chemistries
have been studied or developed.20
Among them are polysulfide-bromide
(PSB) and zinc-bromide batteries
(ZBB). Figure 3 depicts electrochemical
reactions of these two RFBs. The PSB
systems employ electrolytes of sodium
bromides and sodium polysulfides.11,21,36
The electrolyte solutions are separated
by a selective membrane to prevent the
sulfur anions from reacting directly with
the bromine, and the electrical balance is
achieved by the transport of Na+ across
the membrane.21 Polysulfide-bromide
systems with ratings from kWh to MWh
were developed by Regenesys Technologies
Ltd., a wholly own company of
Innogy UK (later bought by VRB, Inc.
and since sold to Prudence Energy). In
ZBB, the electrolyte is an aqueous solution
of zinc bromides plus agents.37,38
During operation, the electrolyte is
pumped through positive and negative
electrode surfaces separated by a microporous
plastic film, or alternatively,
an ionic membrane that selectively allows
the transport of zinc and bromide
but not the aqueous bromine, polybromide
ions, or complex phase. Since zinc
is reversibly deposited from the ions at
the anode, ZBB are not truly redox batteries
and thus often referred as a “hybrid” RFB. In the mid-1980s, Exxon
licensed the technology to a number of
companies that included Johnson Controls,
JIC, who in 1994 sold their interest
to ZBB Energy Corporation. Since
then, ZBB has developed 50-kWh and
500-kWh systems based on a 50-kWh
battery module. Meidisha, another
company that licensed Exxon’s technology,
demonstrated a 1-MW/4-MWh
ZBB battery in 1991 at Kyushu Electric
Power Company in Japan.11
One advantage of PSB and ZBB is
the use of the abundant, low-cost chemicals.
These two batteries have a higher
voltage than VRB and potential higher
energy densities. But their cycle-life,
efficiency, and reliability may be inferior
to VRB. In addition, the formation
of zinc dendrites upon deposition and
the high solubility of bromine in the
aqueous zinc bromide electrolyte has
hindered the ZBB development.19 Its
self-discharge rate is also higher than
VRB and PSB.
With all the stated advantages and
the successful demonstration of systems
up to MWh levels, none of the
RFB technologies have seen broad
market penetration. First and foremost,
the current technologies are still expensive.
Advances in science and technology
continue to bring down the cost;
VRB, for example, is about $500/kWh
or higher,39 which is about two–three
times higher than the target expected for
broad market penetration. The high cost
is directly dependant on the high cost of
materials/components and performance
parameters including reliability, cycle/calendar life, energy efficiency, system
energy capacity, etc.
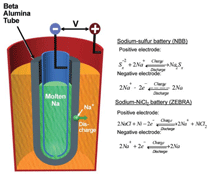
Sodium (Na)-Solid Oxide
Membrane Batteries
Sodium-solid oxide membrane batteries
(SBB) reversibly charge and
discharge electricity via an Na+ conducting
solid oxide membrane. A notable
and the most mature membrane is
Β”-Al2O3 stabilized with Li2O or MgO
that demonstrates an excellent ionic
conductivity.40 To achieve a satisfactory
performance, SBB operate at moderate
temperatures (300–350°C) using liquid
sodium as anodes. The most common
cathode comprises molten S/Na2Sx,
which adds porous graphite felts to
improve its electron conductivity. This
type of battery is known as a sodium-sulfur
(Na-S) battery. Alternatively the
sulfur cathode is replaced by porous Ni/
NiCl2 structures impregnated with molten
NaAlCl4 in ZEBRA batteries. Both
Na-S and ZEBRA are traditionally built
in tubular designs, as schematically
shown in Figure 4, which also depicts
their electrochemical reactions.
The Na-S battery was initially developed
by the Ford Motor Company in
the late 1960s and 1970s for electrical
vehicle applications, and halted in the
mid-1990s with the emergence of battery
technologies such as nickel-metal
hydride and later lithium-ion. By the
early 1980s, the Tokyo Electric Power
Company collaborated with NGK Insulator,
Inc. to develop Na-S technologies
for utility energy storage. By the late
1990s, varied systems up to the MWh
scale had been developed. A number of
MWh systems have been demonstrated
on the electrical grid. The largest system
currently under construction is a 34-
MW/238-MWh (7 hours) Na-S storage
for the Rokkasho wind farm in northern
Japan.
One major advantage of the Na-S
battery is its high energy efficiency (up
to over 90%), due in part to its nearly
100% columbic efficiency. The Na-S
battery demonstrates an energy density
comparable to some lithium-ion chemistries.
In addition, the molten electrodes
in the battery ensure a high current density
and a quick response to changing
power conditions. A calendar life of up
to 15 years and a cycle life of 4,500 cycles
with 90% depth of discharge have
been achieved. However, operation at
elevated temperatures requires an effective
enclosure and/or stringent thermal
management to maintain energy efficiency and provide adequate stand time.
There is also the need to improve safety,
durability, reliability, etc. Molten sulfur
is not a good conductor and corrosive to
the container. A structural breakdown
of the oxide electrolyte would lead to
direct contact of molten sulfur and sodium,
resulting in fire or potential catastrophic
failure. During off-times, the
system must be maintained at elevated temperatures. Freezing cycles induce
mechanical stress, potentially causing
structural failure often after only
a few cycles. The cost is still too high
for broad market penetration, although
technology advancement and scaled
production have reduced it.
In ZEBRA or Na-NiCl2 batteries,
sodium ions are transported through
the oxide membrane from the anode to
the cathode during discharge, reducing
NiCl2 to Ni via migration of sodium
ions in NaAlCl4 The concept of ZEBRA
was proposed in 1978 and further
developed by BETA Research and Development
Ltd. in England.41,42 MESDEA
acquired the ZEBRA technology
and has since been involved in commercialization
efforts. Recently FIAMM
and MES-DEA formed a new company,
FZ Sonick SA, to further develop the
technology. The use of solid or semisolid
cathodes makes Na-NiCl2 batteries
intrinsically safer and less corrosive
than Na-S batteries. The high voltage of
Na-NiCl2 batteries helps energy density.
Nevertheless, there is need of further
improvement in power, reliability, etc.
Recently General Electric developed the
Na-NiCl2 batteries and built a factory in
New York. Under support by the DOE
ARPA-E Program, Eaglepicher is teaming
up with Pacific Northwest National
Laboratory in developing the planar design
of the Na-NiCl2 chemistry.
Lithium-ion Batteries
Lithium-ion batteries store electrical
energy in electrodes made of lithium-intercalation
(or insertion) compounds
(Figure 5). During charge and discharge,
Li+ ions simply transfer across a liquid
organic electrolyte between one host
structure and the other, with concomitant
oxidation and reduction processes
occurring at the two electrodes. The
lithium-ion technologies started with
discovery of intercalation compounds
such as LixMO2 (M=Co, Ni, Mn) that
were initially proposed by Goodenough
and are still widely used today.43,44 The
finding of highly reversible, low-voltage
lithium-intercalation carbonaceous materials
led to the commercialization of
LixC6/Li1-xCoO2 rocking-chair cells by
Sony in 1991.45,46 The lithium-ion cells
operate around 4 V and demonstrate a
capacity and power about 150 Ahkg–1
and over 200 Whkg–1, respectively.46 The favorable electrochemical performance
in energy and power densities
and advancement in system design and
manufacturing made the early lithium-ion
a great success for mobile electronic
applications in spite of remaining challenges.
Among these is that the early
lithium-ion chemistries are inherently
unsafe. The lithiated-graphite electrode
operates at a potential close to that of
metallic lithium, leading to lithium-dendrite
growth and potential electrical
shorting. In the presence of flammable
organic electrolyte solvents currently in
use, there is a risk of heat generation,
thermal runaway, and fire. An additional
challenge is the high cost that may not
be critical to electronic applications, but
is very important for scaled-up vehicle
applications, which so far consider lithium-ion as the most promising technologies.
In the past decade or so, substantial
progress has been made in advancing
the lithium-ion technologies, mainly
driven by broad interests and extensive
efforts for hybrid, plug-in hybrid, and
electrical vehicles. For high-energy capacity, alloys and/or intermetallics
have been extensively investigated as
negative electrodes. While promising
progress has been made with the high-capacity
alloy anodes, structural stability
issues remain that are ascribed to
large-volume expansion during alloying
with lithium.46,47 Metal oxides, especially
lithium titanate spinel Li4Ti5O12,
have been found as safe alternatives to
the graphite anode.48–50 The titanate anode
operates at 1.55 V vs. Li+/Li and can
accommodate lithium with a theoretical
capacity of 175 mAhg–1. While sacrificing
energy density to some extent, the
relatively high potential versus lithium
makes titanate electrodes intrinsically
safer than graphite. In the titanite spinel,
the {Li1/3Ti5/3}O4 framework provides a
three-dimensional network of channels
for facile Li+ diffusion and exhibits little
or no volume expansion even during
lithiation. Accordingly, good reversibility
and the ability to resist structural
change during lithium insertion/extraction
make it an attractive anode for applications
that require a long cycling life.
There are no, or few, side reactions with
electrolytes directly related to the irreversible
capacity and power loss. This
allows for the use of nanostructures to
improve rate capability, and thus power,
without side reactions with electrolytes.
The good chemical compatibility, along
with the relative high potential vs. Li+/
Li make the titanite anode much safer
than the carbon-base ones. In addition
to lithium titanates, varied TiO2 polymorphs
including TiO2-B, anatase, rutile,
etc. have been found as an active
lithium host, and some of their nanostructures
have demonstrated promising
electrochemical properties.51–54
On the positive electrodes, LiNi1/3
Mn1/3Co1/3O2 was developed as an alternative
to the LiCoO2 cathode.55–57
In addition to lower cost, the former
demonstrates higher capacity, longer
cyclability, and better safety compared
to LiCoO2. Other alternatives include
LiMn2O4 (spinel) and its derivatives that
have a voltage of over 4.0 V versus lithium
and a capacity about 10% less than
that of LiCoO2. Although much more
cost-effective than LiCoO2, the spinels
have a tendency to dissolve in electrolyte
and undergo Jahn–Teller-driven
cubic-tetragonal structural distortion
during deep discharge, which degrades
battery performance and reduces battery
life.46,58 In the late 1990s, Padhi et
al. proposed LiFePO4, which exhibits
a lower voltage (~3.5V vs. lithium),
but a higher capacity of 170 mAh/g in
comparison to LiCoO2.59 In addition to
its low cost and being environmentally
benign, the olivine structure is highly
stable and allows for long cycles of Liintercalation/
deintercalation. However,
the material exhibits low lithium-ion
and electronic conductivity. Introducing
nanostructuring, carbon coatings, and
doping shortens the lithium-diffusion
distance and enhances electron conduction,
substantially improving the performance
of the olivine structured chemistry
as cathodes.60–63 Nanostructured
LiFeO4 has gained commercial success
in the lithium-ion batteries.
Given the high energy/power density
and nearly 100% energy efficiency and
anticipated mass production, lithiumion
technologies are considered valuable
storage options for renewable end
users, and distributed grids. There has
been discussion on the use of the lithium-ion battery stacks after their life
service on hybrid or electrical vehicles.
This would extend the values of the batteries
that are initially developed for the
transportation applications. It remains
questionable, however, if the after-use
batteries can meet the performance and
economic matrix for stationary applications.
Alternatively, there are increasing
incentives and the need to develop
lithium-ion batteries specifically for stationary
applications. This becomes particularly
important, given the difference
in requirements between stationary and
transportation applications and the fact
that, currently, no lithium-ion chemistry
meets the performance and economic
matrixes for both applications. The lithium-ion technologies for the stationary
applications should focus on cost-effective
chemistries or materials that provide
long calendar and cycle life. Particular
interest would be on electrode materials
and electrolytes that are structurally
stable and chemically compatible during
lithium insertion/deinsertion. Altairnano
developed a lithium-ion battery
based on nano-titanite anodes and demonstrated
up to 2.0 MW systems. Operating
with a relatively lower voltage
(2.3V) and of a lower energy density
than conventional lithium-ion chemistries,
the Altairnano technology offered
a great range of safety (–40–260°C),
long calendar and cycle life (>15 years
and >10,000 cycles), and high power
(4 kW kg–1). The system demonstrated
a quick response, in a matter of milliseconds,
to control commands and
a round-trip efficiency of about 90%.
However, it could only last up to 15
minutes at the name-tag power. A123
developed a lithium-ion battery based
on nanostructured LiFePO4 cathode and
demonstrated up to 2.0 MW systems
for power management. Similarly, the
A123 system lasted only 15 minutes at
the name power. Heat management appears
among major challenges for better
technologies. Overall, lithium-ion
technologies have not yet been fully
demonstrated to meet the performance
and economic matrix for the utility sector.
Further investment and efforts are
needed to develop suitable lithium-ion
technologies that can support increasing
penetration of renewable energy and
stabilizing of the electrical grid. Significant advancements are needed in materials,
processing, design, and system integration
for the technologies to achieve
broad market penetration.
CONCLUSION
The current trend toward reducing
greenhouse gas emission and increasing
penetration of renewable energy, along
with increasing demands of high-quality
power, calls for urgent development
and implementation of EES. Without
suitable EES, the current electrical grid
could allow for only a limited level of
penetration of renewable energy generated
from intermittent sources such as
wind and solar. Over-penetration would
destabilize the grid, potentially causing
shutdowns and even blackouts. Further
challenging is that intermittent renewable
sources are typically rich in certain
areas, which are often far away from
load centers. Installing EES into the
grid would not only facilitate increasing
penetration of renewables, but ensure
quality power for a society becoming
increasingly digitized. Implementing
EES would help reduce greenhouse gas
emissions by replacing fossil-burning
turbines currently employed to stabilize
the grid. Energy storage can be a key
enabler for a future grid that integrates extensive renewable generation and provides
power for plug-in vehicles. Detailed
studies on effective and economically
viable use of EES in the future grid
are needed.
A number of potential technologies
for EES exist, and some of these have
been demonstrated for utility applications.
However, these technologies are
facing either challenges in meeting the
performance and economic matrix for
the stationary applications, or limits in
environment, site selection, etc. This
calls for both basic and applied research
to further develop current technologies
and to discover new technologies that
can address the needs for renewable and
utility applications.
Currently, only limited R&D have
been performed in advanced storage
technologies for utility and renewable
applications. This is particularly true
compared to storage technology research
and development for vehicle applications.
Unfortunately, there are only
a few government-funded programs
worldwide for developing electricity
storage technologies for stationary applications.
There is a general public and
political lack of awareness of the need
for new technology for these applications.
Even renewable energy industries
are reluctant to lend support due to concerns
about adding extra cost to renewable
power systems as they struggle to
reduce system cost. Lately, however,
there appear signs that the current trend
is reversing. Along with the electrical
storage for vehicle applications, development
and demonstrations of largescale
storage technologies have been
proposed in the American Recovery
and Reinvestment Act of 2009. A number
of other countries have also shown
increasing interest in stationary storage
research and development, suggesting a
bright outlook for development of stationary
energy storage technology for
the future electric grid.
ACKNOWLEDGEMENTS
The authors acknowledge financial
support from the U.S. Department of
Energy’s ARPA-E, Office of Electricity
Delivery and Reliability, and Energy
Efficiency & Renewable Energy (EERE),
along with support by the Laboratory-Directed Research and Development
Program of the Pacific Northwest
National Laboratory (PNNL). PNNL
is a multi-program national laboratory
operated by Battelle Memorial Institute
for the U.S. Department of Energy under
Contract DE-AC05-76RL01830.
REFERENCES
1. R.E. Smalley, MRS Bulletin, 30 (6) (2005). pp. 412–
417.
2. I.E. Agency, Key World Energy Statistics (Paris: International
Energy Agency, 2006).
3. A. Amin and J. Stringer, MRS Bulletin, 33 (4) (2008),
pp. 400–409.
4. C.A. Powell and B.D. Morreale, MRS Bulletin, 33 (4)
(2008), pp. 309–315.
5. V.S. Arunachalam and E.L. Fleischer, MRS Bulletin, 33
(4) (2008), pp. 264–276.
6. P.J. Meier et al., Energy Policy, 33 (9) (2005), pp.
1099–1108.
7. D. Ginley, M.A. Green, and R. Collins, MRS Bulletin, 33
(4) (2008), pp. 355–364.
8. J.P. Holdren, Science, 315 (5813) (2007), pp. 737–
737.
9. B. Hayman, J. Wedel-Heinen, and P. Brondsted, MRS
Bulletin, 33 (4) (2008), pp. 343–353.
10. B.S. Lee and D.E. Gushee, Chemical Engineering
Progress, 104 (3) (2008), pp. S29–S32.
11. Handbook of Energy Storage for Transmission and
Distribution Applications (Washington, D.C.: Department
of Energy and Palo Alto, CA: Electrical Power Research
Institute, 2003).
12. E. Marris, Nature, 454 (7204) (2008), pp. 570–573.
13. Editorial, Nature, 454 (2008) p. 551.
14. L.B.L. Jay Apt and Sompop Pattanariyankool, “Generating
Electricity from Renewables: Drafting Policies
that Achieve Society’s Goals,” in: a white paper prepared
for The National Rural Electric Cooperative Association
(Pittsburgh, PA: Carnegie Mellon University, 2008).
15. 20% Wind Energy by 2030: Increasing Wind Energy’s
Contribution to U.S. Electricity Supply (Washington,
D.C.: U.S. Department of Energy, 2008), www.osti.gov/bridge.
16. Bottling Electricity: Storage as a Strategic Tool for
Managing Variability and Capacity Concerns in the
Modern Grid (December 2008), www.oe.energy.gov/final-energy-storage_12-16-08.pdf.
17. Capital Cost per Cycle of Current Storage Technologies (2008), www.electricitystorage.org.
18. H. Chen, T.N. Cong, W. Yang, C. Tan, Y. Li, and Y. Ding,
Progress in Natural Science, 19 (3) (2009), pp. 291–312.
19. David Linden and T.B. Reddy, editors, Handbook of
Batteries (New York: The McGraw-Hill Companies, Inc.,
2002).
20. M. Bartolozzi, J. Power Sources, 27 (3) (1989), pp.
219–234.
21. C.P. de Leon et al., J. Power Sources, 160 (1) (2006),
pp. 716–732.
22. M. Skallas-Kazacos, M. Rychick, and R. Robins,
“All-vanadium Redox Battery,” U.S patent 4,786,567 (22
November 1986).
23. M. Skyllas-Kazacos et al.,J. Electrochemical Society,
133 (5) (1986), pp. 1057–1058.
24. M. Skyllas-Kazacos, C. Menictas, and M. Kazacos, J.
Electrochemical Society, 143 (4) (1996), pp. L86–L88.
25. M. Kazacos and M. Skyllas-Kazacos, J. Electrochemical
Society, 136 (9) (1989), pp. 2759–2760.
26. E. Sum and M. Skyllas-Kazacos, J. Power Sources,
15 (2-3) (1985), pp. 179–190.
27. G.J.W. Radford et al., J. Power Sources, 185 (2) (2008), pp. 1499–
1504.
28. B.T. Sun and M. Skyllas-Kazacos, Electrochimica
Acta, 36 (3-4) (1991), pp. 513–517.
29. P. Llewellyn et al.,J. Electrochemical Society, 134
(8B) (1987), pp. C458–C458.
30. T. Sukkar and M. Skyllas-Kazacos, J. Membrane Science,
222 (1-2) (2003), pp. 235–247.
31. J.C. Woong, S. Venkataramani, and S.C. Kim, Polymer
International, 55 (5) (2006), pp. 491–499.
32. J.Y. Xi et al., J. Power Sources, 166 (2) (2007), pp.
531–536.
33. J. Zeng et al., Electrochemistry Communications, 10
(3) (2008), pp. 372–375.
34. A. Shibata, S. Kanji, and M. Nakajima, in: IEEE: 1st
World Conference on Photovoltaic Energy Conversion
(Piscataway, NJ: IEEE, 1994), pp. 950–953.
35. O. Hamamoto, G. Okuyama, Z. Kamio, and M. Yoshitake,
in: Proceedings of the Symposium on Batteries and
Fuel Cells for Stationary and Electrical Vehicle Applications (Pennington, NJ: The Electrcochemical Society,
1993), pp. 178–188.
36. A. Price et al., Power Engineering Journal, 13 (3)
(1999), pp. 122–129.
37. K. Cedzynska, Electrochimica Acta, 40 (8) (1995),
pp. 971–976.
38. D.J. Eustace,J. Electrochemical Society, 127 (3)
(1980), pp. 528–532.
39. Bob Johnstone, Discover (October 2008), p. 6.
40. J. Sudworth and A.R. Tilley, The Sodium-Sulfur Battery
(London: Chapman and Hall, 1985).
41. C.H. Dustmann, J. Power Sources, 127 (1-2) (2004),
pp. 85–92.
42. J.L. Sudworth, J. Power Sources, 100 (1-2) (2001),
pp. 149–163.
43. K. Mizushima et al., MRS Bulletin, 15 (6) (1980), pp.
783–789.
44. M.M. Thackeray et al., MRS Bulletin, 18 (4) (1983),
pp. 461–472.
45. M. Mohri et al., J. Power Sources, 26 (3-4) (1989),
pp. 545–551.
46. J.M. Tarascon and M. Armand, Nature, 414 (6861)
(2001), pp. 359–367.
47. R.A. Huggins, in: Lithium Batteries: Science and Technology,
ed. G.-A.Nazri and G. Pistoia (London: Klumer
Academic Publisher, 2004), pp. 270–295.
48. E. Ferg et al., J. Electrochemical Society, 141 (11)
(1994), pp. L147–L150.
49. T. Ohzuku, A. Ueda, and N. Yamamoto, J. Electrochemical
Society, 142 (5) (1995). pp. 1431–1435.
50. Zhenguo Yang, D. Choi, Donghai Wang, Kevin Rosso,
Jason Zhang, Gordon Graff, and Jun Liu, J. Power Sources, 192 (2) (2009), pp. 588–598.
51. G. Armstrong et al., Advanced Materials, 18 (19)
(2006), pp. 2597–2600.
52. D.V. Bavykin, J.M. Friedrich, and F.C. Walsh, Advanced
Materials, 18 (21) (2006), pp. 2807–2824.
53. Y.G. Guo et al., Advanced Materials, 19 (16) (2007),
pp. 2087–2091.
54. D.H. Wang et al., Chemistry of Materials, 20 (10)
(2008), pp. 3435–3442.
55. Y. Makimura and T. Ohzuku, J. Power Sources, 119
(June 2003), pp. 156–160.
56. N. Yabuuchi and T. Ohzuku, J. Power Sources, 119
(June 2003), pp. 171–174.
57. Z.H. Lu, D.D. MacNeil, and J.R. Dahn, Electrochemical
and Solid State Letters, 4 (11) (2001), pp. A191–A194.
58. M. Pasquali, S. Passerini, and G. Pistoia, in: Lithium
Batteries: Science and Technology, ed. G.-A. Nazri and
G. Pistoia (London: Klumer Academic Publisher, 2004),
pp. 313–353.
59. A.K. Padhi, K.S. Nanjundaswamy, and J.B. Goodenough,
J. Electrochemical Society, 144 (4) (1997), pp.
1188–1194.
60. S.Y. Chung, J.T. Bloking, and Y.M. Chiang, Nature Materials, 1 (2) (2002), pp. 123–128.
61. H. Huang, S.C. Yin, and L.F. Nazar, Electrochemical
and Solid State Letters, 4 (10) (2001), pp. A170–A172.
62. K. Striebel et al., J. Electrochemical Society, 152 (4)
(2005), pp. A664–A670.
63. A. Yamada, S.C. Chung, and K. Hinokuma, J. Electrochemical
Society, 148 (3) (2001), pp. A224–A229.
Zhenguo Yang, Jun Liu, Suresh Baskaran, Carl
H. Imhoff, and Jamie D. Holladay are with Pacific
Northwest National Laboratory, 902 Battelle Blvd.,
Richland, WA 99352 USA. Dr. Yang can be reached
at zgary.yang@pnl.gov. |