Recent price fluctuations have focused
attention on the phenomenal increase
of global energy consumption in
recent years. We have almost reached
a peak in global oil production. Total
world consumption of oil will rise by
nearly 60% between 1999 and 2020. In
1999 consumption was 86 million barrels
of oil per day, which has reached a
peak of production extracted from most
known oil reserves. These projections, if
accurate, will present an unprecedented
crisis to the global economy and industry.
As an example, in the United States,
nearly 40% of energy usage is provided
by petroleum, of which nearly a third is
used in transportation. An aggressive
search for alternate energy sources,
both renewable and nonrenewable, is
vital. This article will review national
and international perspectives on the
exploration of alternate energies with
a focus on energy derivable from the
ocean.
INTRODUCTION
As late as 10 years ago, there were
strong proponents on both sides of
the debate about the reality of a rapidly
approaching global energy crisis.
Although all agree that the Earth’s
resources of fossil fuels are finite, the
optimists believe that the resources will
last for a very long time before a crisis
is reached. The world’s appetite for liquid
hydrocarbon as an energy resource
has continued to grow at an exponential
rate. Global output, which was much
less than a million barrels per day in
1900, has increased to 85 million barrels
per day today, growing at a rate
of 1.5–2.0% per year. This supply is
rapidly failing to meet the demand, as
reflected, in part, in the rapid rise and
unprecedented fluctuations of the price
of petroleum. Of all the energy sources
upon which the global economy and industrial
growth are built, the prominent
sources are petroleum, coal, natural gas,
biomass, hydroelectric, and nuclear.
|
HOW WOULD YOU...
|
…describe the overall significance
of this paper?
An aggressive search for alternate
energy sources, both renewable
and non-renewable, is vital. This
article will review both national and
international perspectives on the
exploration of alternate energy with
special focus on energy derivable
from the ocean.
…describe this work to a layperson?
We have almost reached a peak
in global oil production. Total
world consumption of oil will rise
by nearly 60% between 1999 and
2020. These projections, if accurate,
will present an unprecedented
crisis to the global economy and
industry. As an example, in the
United States nearly 40% of energy
usage is provided by petroleum,
of which nearly a third is used in
transportation.
|
A seminal paper, published in 1956
by an American petroleum geologist,
M. King Hubbert,1 now popularly
known as “Hubbert’s peak,” was highly
criticized at the time by his peers. Utilizing
a statistical model, Hubbert predicted
that the production of oil would
reach a peak in the lower 48 states of
the United States, after which it would
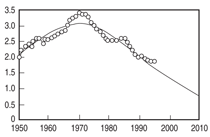
diminish. Figure 1 illustrates Hubbert’s
prediction superimposed with the actual
data since his publication.
Validation of the accuracy of this
model has led to a critical evaluation
by a number of agencies, including the
U.S. Department of Energy (DOE), to
predict a global peak in production. A
Saudi oil geologist, S.I. Al Husseini,
reported the results of his study over
10 years of the 250 major oil fields that
provide most of the world’s oil. Since
2004 the production from these fields
has reached a plateau of 85 million
barrels of oil per day. This flat production
rate, he claims, may last as long
as 15 more years, after which there
will be a gradual decline, suggesting
that the world has already reached a
peak in production. This projection is
consistent with those of the head of
the French oil company, Total, and the
chief executive officer of Conoco Phillips.
To meet the increasing demands
by 2010, they project that nearly 40%
of the daily oil needs will have to come
from either untapped or undiscovered
oil fields. Table I illustrates the rate of
world oil consumption since 1975 and
projected to 2015 in the industrialized,
Eastern European, and developing nations.
The figures clearly indicate that
the rate of increasing demand from
developing nations, primarily China
and India, outpaces those in the other
regions.
Table I.
The Rate of World Oil Consumption Since 1975 and Projected to 2015 in the
Industrialized, Eastern European, and Developing Nations*
|
| Region |
1975 |
1985 |
1995 |
2005 |
2015 |
| Industrialized |
38.0 |
35.5 |
42.5 |
48.0 |
53.0 |
| EE/FSU |
8.5 |
11.0 |
6.0 |
7.5 |
9.5 |
| Developing |
8.0 |
13.0 |
21.0 |
32.0 |
43.0 |
* in million barrels per day
|
ALTERNATE ENERGY SOURCES
Conserving energy through improved
technologies, such as hybrid
automobile engines, use of electric
power and hydrogen, and improved turbine
designs, would assist in reducing
fossil fuel consumption rates. However,
these developments will be slow in
coming and will not offset the projected global growth rates of energy consumption.
Alternative energy resources can be
broadly divided into two groups: renewables
and non-renewables. The renewables
include biomass, hydrogen,
wind, nuclear fusion, solar terrestrial,
ocean waves and tides, geo- and ocean-thermal,
hydroelectric, and synthetic
fuels. Some of these approaches are
limited by geographic locations and
economic factors. These include geo and
ocean-thermal, hydroelectric,
waves, and tides. Other resources are
actively pursued by many countries to
produce various forms of hydrocarbons
for use either as additives to the existing
fossil fuels or as a fuel substitute.
The non-renewables predominantly include
clean coal, nuclear fission, synthetic
gas, oil shales, and methane hydrates.
The innovative technology demands
and cost factors associated with
effective utilization include sequestration
of gaseous and solid byproducts,
radioactive waste disposal, and environmental
issues.
Blomass
Energy extracted from biomass dates
back to very early human activities,
with the discovery of fire, and continues
to be the world’s fourth-largest energy
source, providing 5 × 1019 joules
per year. This energy source is currently
derived from agricultural waste, forestry
waste, municipal solids, industrial
waste, and energy crops. The goal in
the United States, reported by the National
Renewable Energy Laboratory of
the DOE,2 is that by 2020, 10% of
transportation fuels, 5% of electric
power production, and 18% of chemicals
and materials will be provided
from biomass sources. Research and
development efforts are underway to
maximize biomass-derived methanol,
ethanol, and diesel from sugar cane,
cassava, soybeans, corn, jatropha, miscanthus,
and switch grass, amongst
others, with claims of ethanol production
varying from 3,300 to 14,000 liters
per hectare.3 Liquid hydrocarbon production
from sugar cane has been successfully
demonstrated by Brazil. The
crop is confined to specific tropical and
equatorial regions of the world, providing
a limited supply either as a substitute
for or as an additive to petroleum.
In the United States, major efforts are
in progress to augment petroleum with
as much as 10–15% ethanol derived
from corn. This effort has led to numerous
controversies, including:
- Higher energy cost for corn production
and conversion to ethanol
- The energy equivalent of one liter
of gasoline requires 1.5 liters of
ethanol
- To provide 10% of all auto and
truck transportation fuel needs
from ethanol will require about
8 × 107 hectares of agricultural
land to be removed from food production
- The hygroscopic property of ethanol
will contribute to accelerated
corrosion in fuel containers and
potential ice formation when the
fuel is exposed to lower temperatures
- Ethanol has a significantly lower
flash point than JP5 and JP8 jet fuels
The most controversial debate on
ethanol production concerns the use of
grain as feedstock. The energy requirement
for growing the corn, harvesting
it, transporting it, and manufacturing
the ethanol versus the energy content of
ethanol has been the center of this controversy.
A number of papers have been
published suggesting that the net energy
value, which is the energy content of
ethanol minus the fossil energy used to
produce ethanol, varies from –9.4 × 106
joules per liter to 8.5 × 106 joules per
liter.4 Table II provides the energy content
of various biofuels in comparison
to regular unleaded gasoline and diesel
fuel.
Table II. Gasoline Equivalents
|
| Fuel Type |
MJ per Liter |
Liter Equivalent |
| Gasoline, Regular Unleaded (typical) |
32 |
1.00 |
| Gasoline, Reformulated (10% MBTE) |
31 |
1.02 |
| Diesel (typical) |
36 |
0.88 |
| Methanol (M-100) |
16 |
2.01 |
| Ethanol (E-100) |
21 |
1.50 |
| Biodiesel (B-20) |
36 |
0.88 |
|
Material challenges for biomass fuel
substitutes include the requirement for
corrosion-resistant materials such as
stainless-steel- or titanium-based alloys
and antifouling paints. Biodiesel, extracted
from sources such as vegetable
oils, animal fat, and recycled oil, presents
yet another set of challenges for
fuel usage and materials, including
moisture uptake, tank corrosion, degradation
during prolonged storage, biofouling,
poor lubricity, decreased energy
per liter, and gelling at low temperatures.
The technological challenges are
easily resolved by use of appropriate
existing materials and additives. Biodiesel
has the potential for recycling vast
amounts of recyclable oils and animal
fats. (In the United States alone 19 ×
109 kilograms of animal fat are produced
per year.)
Hydrogen: A Clean Energy Source
Some of the major advantages of hydrogen
as an energy source include its
abundance, high energy content per
mass, environmental neutrality as an
energy source, excellent flammability,
flame speed and auto ignition temperature,
and potential for use in fuel cells.
Hydrogen can be derived from sources
such as biomass, electrolysis using energy
from hydro, wind, solar, or nuclear,
and, with appropriate carbon sequestration,
from oil, coal, and natural
gas.
Three of the most viable approaches
to hydrogen production are steam reforming
of methane, the use of electricity
to produce hydrogen from water,
and the gasification of coal. Recognizing
the shortage of CH4 and its rapidly
increasing cost, large-scale production
of hydrogen by this approach would be
prohibitive. For electrolysis, there will
be a need to use 3.9 kWh of electrical
energy to produce 1 m3 of hydrogen
that will provide an energy value of 3.2
kWh (80% efficiency). Coal gasification is a well-known commercial process
and has been in the marketplace
since 1970. Coal is an attractive hydrogen
feedstock because of its abundance
in the Earth’s crust and coal mining infrastructure
already in place with a
price structure that is low and non-volatile.
The major challenge in hydrogen
production from coal would be to sequester
carbon dioxide. One possibility
is the use of deep saline aquifers with
good top seals that are estimated to be
able to store as much as 4.5 × 1013 kilograms
of carbon.5
Apart from nuclear energy sources, a
number of energy harvesting technologies
could be considered for production
of hydrogen. Utilization of the sun’s
energy by photonic processes is extensively
being examined, utilizing organic
and inorganic photovoltaic materials.
The peak solar flux on Earth is about
1,000 W/m2 with an average (considering
day, night, and solar angle) of 200
W/m2. With the current conversion efficiency
of about 6%, the solar field required
to produce the necessary amount
of hydrogen to replace liquid hydrocarbon
transportation fuel alone would
cover 1 × 107 hectares.6
The DOE, along with the European
Union countries, has embarked on the
challenge of what is known as the hydrogen
economy. This activity has been
identified for development in several
phases. The first phase consists of developing
the technology needed for hydrogen
power and transportation systems
for selected locations; the second
deals with market penetration and commercialization;
the third, development
of large-scale infrastructure; and the
fourth, development of a national and
international infrastructure. The critical
path for developing a hydrogen economy
has been targeted by the DOE based
on performance and cost reduction.
The targets are 2–3x performance increase
for lightweight compact hydrogen
storage, 4x cost reduction for hydrogen
production compared to conventional
fuels, and 10x cost reduction
in carbon sequestration. All of these
challenges or targets create significant
needs for advanced material development.
Among those needs are, for example,
for distribution pipelines, use of
expensive ferrous and titanium alloys
immune to hydrogen embrittlement, effective
metal in combination with fiber reinforced
composite tanks for hydrogen
storage at 69 MPa, development of
high-temperature materials for effective
engine performance in ground
transportation systems, and enhanced
performance of hydrogen sensors for
leak detection. (Irrespective of improved
pipelines and hydrogen delivery
at pumping stations, it is estimated that
1–3% of hydrogen will leak into the atmosphere.
Extensive leakage will cause
hydrogen escape into the troposphere
that will deplete the OH that serves as a
scrubber of atmospheric contaminants
and greenhouse gasses.)
One of the major materials challenges
is to safely store hydrogen. Apart
from storage of hydrogen under pressure
in sustainable containers, three
major approaches are under consideration
for hydrogen storage: reversible
metal hydrides, non-reversible chemical
hydrides (hydrogen carriers), and
advanced adsorbent materials. The key
challenges are lowering of desorption
temperatures, improving kinetic response,
and providing proper heat management
during refill, decreasing regeneration
costs, improving volumetric
capacities, and developing manageable
desorption temperatures. Metal hydrides
appear to provide the highest
percentage of hydrogen weight fraction
in Mg(BH4)2(NH3)2 and Mg(BH4)2 with
12% hydrogen weight fraction. However,
these compounds require ~350°C
for hydrogen release. The targets for
advanced material development are to
optimize storage capacity at low hydrogen
uptake and release temperatures,
while maximizing the life cycle (durability)
and minimizing costs.
Additionally, it is important to recognize
that hydrogen at 69 MPa pressure
has 1/5 the energy density of gasoline.
Liquid hydrogen increases the ratio
to 1/4.6 Before hydrogen can be used
widely for various energy applications,
its wide limits of flammability, low
spark ignition energy, nearly invisible
combustion flame, and high cost of
pipelines (4 × 105 euros per kilometer)
would continue to pose serious challenges.
Coal to Liquid
Another technology that is being
heavily pursued is the production of
liquid fuel from coal using the well-known
Fischer–Tropsch process.7 The
process primarily consists of gasifying
coal and using the water gas shift reaction
to produce appropriate concentrations
of carbon monoxide and hydrogen
using conventional catalysts such as
iron-, cobalt-, and nickel-based powders
at modest temperatures of 150–200°C and modest pressures of about
1 MPa. The process provides gasoline
and diesel fuel and, depending on the
pressure temperature combination,
CH4. However, the process produces
excess carbon dioxide and, based on
the chemistry of the coal used, various
other pollutants. Since the invention of
this process at Kaiser Wilhelm Institute
in the 1920s, it has been significantly
improved by Sasol in South Africa,
which is in the process of exporting the
technology to China with a production
capacity of 80,000 barrels of oil per
day. The process is now being extended
to use biomass and natural gas as feedstocks.
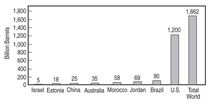
Oil Shales
Sedimentary rocks called oil shales
contain significant amounts of hydrocarbons
and are distributed world wide
including in countries like the United
States, Australia, China, Sweden, and
Estonia. There are also known deposits
in France, Germany, Brazil, Mongolia,
and Russia. Figure 2 illustrates the
global distribution of oil shale.
By far the largest deposit, consisting
of more than 70% of the world
reserves, is located in the Green River
Basin of the Colorado River, covering
parts of Colorado, Utah, and Wyoming.
The total reserve is estimated to be in
excess of 1,500 billion barrels of oil.8,9 Pyrolizing the oil shales can transform
kerogen based oil into synthetic crude
oils. The organic matter in the oil shale
contains an atomic ratio of hydrogen to
carbon at approximately 1.5 to 3 times
higher than coal.10 This reserve is significantly
greater than the proven estimate
of world crude oil reserve. The conventional
methods of extracting crude oil
from oil shales include surface and underground
mining. There are two technologies
utilized for extraction of oil.
The retort process involves heating the
shale in an oxygen-free environment
at temperatures between 450°C and
500°C, which decomposes the kerogen
into gas, oil, and solid residue. The in
situ decomposition process involves
heating the deposit underground and
subsequently extracting the oil and gas
from the decomposed product. Current
costs for extracting a barrel of oil from
oil shales are estimated to be from $40
to $60 per barrel. There are a number
of aspects that have significant environmental
impact, including acid drainage,
sulfur gas emission, atmospheric pollution,
demands for water and electric
power, disposal of spent materials and
arsenic-bearing chemicals in the leach
deposits, the need for hydrogen for upgrading
the chemistry to produce suitable
hydrocarbons, and regulating carbon
emissions to meet regulatory standards.11 Over the last 80 years, Estonia
has met most of its energy demand with
oil shales.
Tar Sands
Tar sands, often referred to as bituminous
sands or oil sands, exist in many
countries, with exceptionally large
quantities in Canada and Venezuela.12 Extraction of oil from tar sands has
been performed for nearly 200 years
and has been used to obtain coal gas
for heating and lighting.13 Other countries
with reported deposits include the
United States, Middle Eastern countries,
Russia, Canada, and Venezuela.
More than 40% of the Canadian oil production
in 2007 came from tar sands.14 The estimated reserves in Canada are
about 1.7 trillion barrels, about 20% of
which is exported to the United States.
Most of the Canadian deposits are in
northern and northeastern Alberta. Although
the extraction of crude from tar
sands is much simpler than extraction
from oil shales, crude from tar sands is
usually too heavy for pipeline transportation
as is and is currently transported
by pipeline only after an emulsification
process with 30% water. The crude has
very high sulfur content and presents
challenges in terms of environmental
concerns, including disposal of toxic
chemicals, wastewater drainage to rivers,
and carbon dioxide emission, as
well as deforestation. Two tons of tar
sands produce one barrel of oil with an
extraction of about 75% of the bitumen.
Ample availability of water, about 3 to
4 times in volume for each unit of crude
oil, is a requirement for oil synthesis.15 Table III provides a comparison of the
principal factors influencing the extraction
of crude oil from tar sands and oil
shales.
Table III.
Comparison of Principal Factors Influencing Economics of Producing Crude Oil
|
| Characteristic |
Tar Sands |
Oil Shale |
| Reserves |
>1 trillion bbl |
>1 trillion bbl |
| Grade (Richness) |
25 gallon bitumen / ton |
25 gallon kerogen / ton |
| Hydrogen Content |
10.5% |
11.8% |
| N and S Removal |
6.2 wt.% |
4.0 wt.% |
| Loss to Coke |
33 lb / ton of ore |
Nil (burned for energy) |
| Net Yield of Oil |
0.5 bbl / ton mined |
0.58 bbl / ton mined |
|
Wind Energy
The extraction of energy from the
wind is well developed and currently
provides over 90 gigawatts of energy
worldwide,16 amounting to less than
1% of the world’s electricity consumption.
Countries such as Spain and Denmark
have been aggressively engaged
in wind power. As recently as during
the last five years, European countries,
the United Kingdom, and China have
led the world in developing offshore
wind power, particularly in the areas of
the North Sea and the Baltic Sea.17 In
terms of wind power capacity, the United
States leads the world at nearly 17
megawatts in 2007.18 The effective usage
of wind power is highly location dependent,
requiring wind speeds in
excess of 7 meters per second, usually
only available in high elevation or offshore
locations. Although this technology
is growing rapidly, it comes with a
number of problems, such as connectivity
to the existing grid, or utilization
to store energy on location as hydrogen
by electrolyzing water. The grids have
to be designed to carry excess electric
loads during high winds. Other problems
relating to large wind farms, particularly
those located near urban regions,
are that they contribute to radar
clutter for the aviation industry, requiring
stealthy wind turbine design. Additionally,
concerns have been expressed about protection of wildlife,
such as migratory birds running into the
turbine blades and sea life from the
acoustic noise. Enhancing the power
production beyond 750 kilowatts to
2 megawatts per wind turbine requires
improved turbine design with turbine
blades greater than 100 meters long.
These blades demand higher stiffness
and resistance to fatigue and environmentally
imposed degradation such as
corrosion and stress corrosion, particularly
for those installed offshore. Various
approaches to resolve these issues
are under consideration.
Ocean Energy
Extraction of energy from tides resulting
from twice-daily variations in
the sea level due to the gravitational effects
of the sun and the moon, as well as
from wave motion in the littoral regions
of the seas, has been examined for potential
energy production. Tidal energies
can be efficiently extracted from
only about 40 sites worldwide, which
imposes significant limits for utilization.
This approach, exploited by countries
like France and Norway, is highly
cost-intensive and imposes several environmental
and navigational issues.
Although the technology is well known,
there are a number of materials-development
challenges to enhance efficiency.
Wave energy, on the other hand, has
significant potential, although it is limited
to specific coastal regions of southern
parts of South America, Australia,
and the United Kingdom. Several research
projects in the United States,
most prominently those supported by
the Naval Facilities Engineering Command,
are developing various test systems
to produce 1 kilowatt from a single
buoy. Although this technology has the
potential of producing 50 kilowatts
from a single buoy, the approach has a
number of disadvantages that include
serious operational problems during
high sea states, impediments to coastal
navigation and fisheries, and technological
issues to reduce capital investment.
Yet another approach to extracting
energy from the ocean is to take
advantage of regions where large thermal
gradients exist between the surface
water and deep water.19 This effort is
still in the research and development
stage, primarily supported by the U.S.
Navy to evaluate the efficiency of this
approach and identify appropriate locations.
Extensive studies in the United
States and Japan are under way to develop
a 10–12 megawatt ocean thermal
energy system. The challenges for this
approach confine energy extraction to
equatorial and tropical regions of the
ocean where the sea state fluctuations
and ocean currents are not significant
enough to disturb the energy extraction
process. Materials for wide diameter
pipelines to depths of nearly a thousand
feet present a critical challenge to the
stability of the surface platform, which
would need to withstand abrasion, corrosion,
and fouling. If successful, the
generated power could either be directed
to produce hydrogen or to provide
electrical power for liquid hydrocarbon
production through downscaled Fischer–Tropsch plants.
Solar Energy
Most of the Earth’s energy resources,
either in stored energy form in biomass,
fossil fuel, and methane hydrates, or delivered
each day from the sun, are derived
from the sun’s energy. The sun’s
energy impacting the Earth in a given
day is equivalent to energy consumption
over seven years. Conversion of
solar energy to electricity using photovoltaic
cells or solar energy concentrators
has been the topic of keen research
and development interest for more than
60 years. European countries, Japan,
and the United States have led the development
of photovoltaic technology
for electricity production through the
utilization of silicon-based solar cells.
This technology is rapidly evolving to
produce multi-megawatt systems in
various regions of the world. Research
is aggressively progressing in improving
efficiency and cost by advancing
thin photovoltaic technologies and solar
energy concentrators. Organic photovoltaics
have demonstrated significant
improvement in efficiency over those of
crystalline and amorphous silicon, although
their long-term stability and robustness
is yet to be realized. Another
area of very high efficiency solar cells
based on GaAs multilayer solar cells is
under intense investigation. These devices
will have niche applications but
because of high cost will not penetrate
the large commercial market. Solar
thermal systems have the unique advantage
of storing the heat from solar radiation
in high-temperature fluids up to
values greater than 400°C for use in turbines
to generate electric power. This
approach presents a number of material
challenges, such as long-term stability
of alloys in corrosive fluids, environmentally
stable reflective mirrors, and
long-term stability of low-cost heat exchanger
systems.20 Some of the major
disadvantages in development of large
solar fields relate to their geographic
location where the solar energy flux is
high and the energy conversion efficiency
of the system is many times
greater than the current 6% to 7%. High demand for energy on Earth in regions
where the average solar flux is well below
200 watts per square meter brings
an additional challenge for appropriate
coupling with the existing grid system
and transportation of power to high demand
urban regions.
Methane Hydrates: An Abundant
Clean Energy
There is an abundance of methane
stored as methane hydrates (clathrates)
along the continental margins and permafrost
regions of the Earth. Although
the existence of these solids at low temperature
and elevated pressure has been
known for some time, it was discovered
in the mid-1930s that methane hydrates
crystallized as solids above 0°C in gas
pipelines.21 It is estimated that more
than half of the organic carbon in the
Earth’s crust exists in the form of methane
hydrates deposits (nearly 1 × 1016
kilograms of carbon equivalent). All
the recoverable and non-recoverable
fossil fuels (coal, oil, and natural gas)
consist of a quarter of the carbon distribution.
The remaining quarter is widely
distributed amongst waste materials,
peat, soil, animals, and dissolved organic
matter in the oceans. The origin
of these hydrate deposits is due to microbial
decomposition of organic matter
at appropriate temperatures and
pressures. These deposits, illustrated in
Figure 3,22 dissociate back into water
and methane below the sea floor due to
an increase in geothermal temperature
gradient and, under the proper conditions
of the sediment structure, remain
in large cavities.
In the Gulf of Mexico and along the
Cascadia Margin and the Blake Ridge
of the United States and Nankai Trough
off the coast of Japan and the Andaman
Sea off the coast of India, about 1014
cubic meters of gas are believed to be
present.23 Recent studies have uncovered
vast deposits in the permafrost regions
of Alaska and Canada. Technologies
dealing with drilling in the continental
margins, which are typically at
greater depths of the water column,
along with improved understanding of
the physics and structure of ocean sediments,
are needed to exploit this vast
resource, which can either be used directly
in ground transportation systems
with minor modifications to the internal
combustion engine, or converted to
liquid by the Fischer–Tropsch process
or to steam, reforming the gas to produce
hydrogen.
Deep Carbon Cycle
A highly controversial hypothesis
has been under debate on the subject
of deep carbon reservoirs in the Earth’s
subsurface crust and core. A workshop
on deep carbon cycle was organized
by the Geophysical Laboratory of the
Carnegie Foundation during 2008.24 The hypothesis contends that large carbon
and hydrogen fluxes emerge from
the Earth’s core to the upper strata,
where they transform to form alkanes
resulting from rapid drop in pressure
and temperature. The debate includes
issues related to the extent of deep carbon
reservoirs in the Earth’s core, magnitude
and kinetics of carbon flux to the
upper crust, and on abiotic organic synthesis.
While these issues are intriguing
and scientifically challenging, the
origins of deep hydrocarbons remain to
date unknown
CONCLUSIONS
It is essential that, during the early
part of the 21st century, humankind
wean itself from addiction to fossil fuels,
primarily petroleum, which has
been the main source of industrial
growth during the last century. A number
of alternate energy production approaches,
both renewable and non-renewable,
such as coal, natural gas,
methane from methane hydrate, biomass,
fission and fusion, hydroelectric,
solar, wind, ocean, thermal, and geothermal,
are potential candidates that
could collectively replace the need for
rapidly depleting fossil fuel reserves in
the Earth’s crust.
ACKNOWLEDGEMENTS
The author wishes to acknowledge
contributions from several of his colleagues
including Dennis Hardy, Joe
Gettrust, and Rick Coffin. Special gratitude
is expressed for the assistance of
Steve Gill for his patience in the preparation
of the manuscript and assistance
in the collection of references.
REFERENCES
1. M.K. Hubbert, “Nuclear Energy and the Fossil Fuels”
(Presentation at the Spring Meeting of the Southern
District, American Petroleum Institute, San Antonio, TX,
7–9 March 1956).
2. R.E. Armstrong, “From Petro to Agro: Seeds of a New
Economy,” Defense Horizons, 20 (Washington, D.C.:
National Defense University, 2002).
3. L.R. Brown, Plan B: Rescuing a Planet Under Stress
and Civilization in Trouble (Washington, D.C.: Earth
Policy Institute, 2006).
4. H. Shapouri et al., The Energy Balance of Corn Ethanol:
An Update, Agricultural Economic Report No. 814 (Washington, D.C.: U.S. Department of Agriculture, Office
of the Chief Economist, Office of Energy Policy and
New Uses, 2002).
5. D.R. Hardy et al., DoD Future Energy Resources
Workshop Proceedings (Washington, D.C.: Office of
Naval Research, the Naval Research Laboratory, the
National Energy Technology Laboratory and the National
Defense University, 2003).
6. T. Coffey et al., “Hydrogen as a Fuel for DoD,” Defense
Horizons, 36 (Washington, D.C.: National Defense University,
2003).
7. Fischer–Tropsch archive, www.fischer-tropsch.org.
8. Survey of Energy Resources, ed. 21 (London: World
Energy Council, 2007), pp. 93–115; www.worldenergy.org/documents/ser2007_final_online_version_1.pdf.
9. Annual Energy Outlook 2006, www.eia.doe.gov/oiaf/archive/aeo06/pdf/0383(2006).pdf.
10. Arvo Ots, Energetika (Lithuanian Academy of
Sciences Publishers) 53 (2) (2007), pp. 8–18.
11. A.K. Burnham, “Slow Radio-Frequency Processing
of Large Oil Shale Volumes to Produce Petroleumlike
Shale Oil” (Livermore, CA: Lawrence Livermore
National Laboratory, 2003), https://e-reports-ext.llnl.gov/pdf/243505.pdf.
12. “Alberta’s Oil Sands: Opportunity, Balance”
(Alberta, Canada: Government of Alberta, March
2008), www.environment.alberta.ca/documents/oil_sands_opportunity_balance.pdf.
13. “Coal Tar,” City of Kingston, Ontario, Canada
(2007), www.cityofkingston.ca/residents/environment/coaltar/index.asp.
14. Canadian Energy Overview 2007 (Calgary, Canada:
National Energy Board of Canada, May 2007), www.neb.gc.ca/clf-nsi/rcmmn/hm-eng.html.
15. John Laumer, “Alberta Tar Sands: A North American
Overview,” Treehugger (28 January 2006), www.treehugger.com/files/2006/01/alberta_tar_san.php.
16. Global Wind Energy Council News (Brussels,
Belgium), www.gwec.net/index.php?id=28.
17. “Global Wind Power Market Potential,” Energy
Business Reports (Dublin, Ireland: Research and
Markets, January 2007), www.researchandmarkets.com/reports/c50505.
18. “Installed Wind Power Capacity Surged 45% in
2007,” American Wind Energy Association Market
Report (Washington, D.C.: AWEA, 2007), www.awea.org/newsroom/releases/AWEA_Market_Release_q4_011708.html.
19. “Deep Pipelines for Ocean Thermal Energy
Conversion,” Makai Ocean Engineering (Kailua, Hawaii:
2007), www.makai.com/p-otec.htm.
20. David Grinley et al., MRS Bulletin, 33 (4) (2008),
p. 355.
21. E.G. Hammerschmidt, Ind. Eng. Chem., 26 (1934),
p. 851.
22. B.B. Rath, Advanced Materials for Energy
Conversion II, ed. D. Chandra, R.G. Bautista, and L.
Schlapbach (Warrendale, PA: TMS, 2004), pp. 19–29.
23. T.S. Collett, 1995 National Assessment of United
States Oil and Gas Resources, U.S. Geological Survey
Digital Data Series 30, ed. D.L. Gautier et al. (Reston,
VA: U.S. Geological Survey, 1995).
24. Deep Carbon Cycle Workshop Summary Report (Washington, D.C.: Carnegie Institution, Geophysical
Laboratory, May 2008), www.gl.ciw.edu/workshops /sloan_deep_carbon_workshop_may_2008.
Bhakta B. Rath is associate director of research at
the Naval Research Laboratory, 4555 Overlook Ave,
SW, Washington, D.C. 20375. |