Disclaimer: The material in this paper is intended for
general information only. Any use of this material in
relation to any specific application should be based
on independent examination and determination of
suitability for the application by professional qualified
personnel. Those making use of or relying upon the
material assume all risks and liability arising from such
use or reliance.
Nanomaterials promise to transform
commercial products, providing significant
societal benefits. As the nanomaterials
enterprise grows, so too, does
the debate over environmental, health,
and safety (EH&S) aspects of producing
them. This article discusses nanomaterial
EH&S needs and progress being
made by industry, academic institutions,
and government laboratories.
INTRODUCTION
The following presentations from NanoMaterials 2007 addressed the topic of
environmental and health issues. Click on a title to view the powerpoint presentation.
|
The long-predicted technological
and societal impacts of nanomaterials
are starting to be realized. Nanomaterial
programs continue to be funded
at local and national levels around
the globe.1 The United States invests
approximately $1.4 billion per year,2
with global funding being three times
greater.3 Nanotechnologies are attracting
new students to science and
engineering. Universities are offering
“nano” curricula, ranging from lectures
to new degrees.4 Improved electronics
and high-performance coatings are being
developed and commericialized.5
These are among the positive impacts
of nanotechnology.
Environmental, health, and safety
(EH&S) issues of producing nanomaterials,
however, remain a concern among
certain segments of the technical community and the general public.6–13 Here
is a glimpse at the ongoing dialogue:
“The most attractive properties of
nanomaterials for medical and technological
applications, including their
small size, large surface area, and
high reactivity, may also lead to new
and unusual toxicity.” 6
- “Nanotechnology–Toxicological
Issues and Environmental Safety”
NATO Advanced Research Workshop
“While it is by no means certain that
emerging nanotechnologies will present
significant risk, we can be sure
that inaction in addressing risk will
pave the way to public distrust and the
potential for serious harm to occur.” 7
- Andrew D. Maynard,
Woodrow Wilson International Center
for Scholars
“Risks posed by nanomaterials, like
risks posed by chemicals, cannot be
easily generalized. Both hazard and
exposure potential will vary widely
for different nanomaterials and for
products or applications that incorporate
nanomaterials.” 8
- “Environmental, Health and Safety
Research Needs for Engineered
Nanoscale Materials”
National Nanotechnology
Coordination Office
“The urgency of nano-EHS research
affects the entire NNI (National Nantechnology
Initiative) investment . . .
(as) fewer of these transformative
technologies will make it into commerce
if the technology transfer pipeline
becomes clogged by concerns
about nanoproduct safety.” 9
- Vicki L. Colvin,
International Council
on Nanotechnology
“Insurers would be prudent to consider
adverse scenarios (to human health) when agreeing terms and conditions
and . . . whether to exclude
losses due to the reduction of property
value (resulting from environmental
exposure).” 10
- “Nanotechnology Recent
Developments, Risks and
Opportunities,”
Lloyds, London
In 2008, more than $58.6 million
will be spent in the United States on
nanomaterials EH&S issues.2,3 Work
ranging from characterization and detection
to risk assessment and communication
will be funded. The fact
remains, however, that nanomaterials
EH&S knowledge lags commercial development
of them. A systematic, coordinated
approach is therefore needed
to provide valuable risk management
guidance while avoiding broad sweeping
statements not based on fundamental
scientific methods. Concurrently,
current EH&S best practices must be
disseminated throughout the growing
nanomaterials enterprise.
This article builds upon the Environmental,
Health and Safety forum at
Commercialization of NanoMaterials
2007 to highlight nanomaterials EH&S
issues.14–19 Also, references to current
EH&S best practices are presented to
aid ongoing research, development, and
commercialization efforts for nanomaterials.
Links to the presentations from
the EH&S forum are provided in the
sidebar.
NANOPARTICLES:
ENVIRONMENTAL IMPACT
AND HUMAN EXPOSURE
Once released into the environment,
nanoparticles cannot easily be reclaimed.
Nanosized particles can disperse
quickly in air. Gravitational settling in water may be slowed for certain
nanoparticles, potentially leading to increased
contact with aquatic species.
Speculation exists that nanoparticle effluents, both water-borne and atmospheric,
could contaminate soil and
groundwater, thus spreading into the
water cycle, vegetation, and crops.20 As
such, ecological concerns ranging from
direct contamination of food chains to
crop yield are being investigated.
Human exposure to nanoparticles
can occur via inhalation, skin content
(dermal absorption), injection, and ingestion.
The retention of nanoparticles
in the body and any potential detrimental
effects need to be determined. Much
work is needed, though, to quantify the
transport and behavior of nanoparticles
in living systems.
Toxicological studies on ultra-fine
and nano-sized particles have mainly
focused on the respiratory system. Particulate
matter, especially fine particles
(2.5 μm in diameter and smaller), can
deposit throughout the respiratory tract,
even deep in the alveolar portions of
lungs. Water-soluble particles can rapidly
pass into the blood stream and
translocate to other organs. Furthermore,
lipid soluble particles may be
retained in the lungs for months or even
years.14
CLOSING THE GAP IN
NANOMATERIALS EH&S
KNOWLEDGE
Numerous recommendations exist
on addressing nanomaterials EH&S
needs.8,19–25 Common among all of
these are: nanomaterials characterization,
monitoring, dose-response nanotoxicity
studies, worker safety protocols,
and risk assessment.
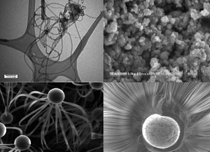
Nanoparticle behavior in the body
cannot be generalized. As shown in
Figure 1, the aspect ratio and surface
morphology of nanoparticles can vary
substantially, potentially contributing
to differences in nanotoxicity. Furthermore,
the state of the nanoparticle
greatly affects EH&S performance.
Different behavior is observed for particles
in liquid, in a solid matrix, or in
dry particulate form.16 Metallographic
evaluations must therefore be conducted
to properly categorize nanoparticle
and nanomaterial toxicity.
Monitoring laboratory and production
environments is important for collecting
meaningful EH&S data. The
American Chemistry Council has published
a “reasoned approach to testing
nanoscale materials” to aid materials
producers.26 Direct-read instruments
can quantify worker exposure to
nanoparticles if compensation is made
for naturally occurring nanoscale particles.15,17 In addition to individual research
projects and corporate industrial
hygiene programs, the U.S. National
Institute of Occupational Safety and
Health (NIOSH) is conducting field
studies in order to develop dose-response
relationships.19,21
Engineering environmental controls
and personal protective equipment
(PPE) for handling nanomaterials are
also being investigated. High-efficiency
particulate air (HEPA) filtered vacuum
systems appear to be effective for
removing airborne nanoparticles.14,16
The National Institute of Occupational
Safety and Health is currently conducting
nanoparticle PPE studies.19 This
work has shown filtration media follows
classical single-fiber theory down
to 4 nm. Ongoing efforts are quantifying
respirator seal effectiveness for exposure
to 5 to 400 nm particles and
penetration through PPE fabrics.
In 2005, NIOSH completed a thorough
dose-response study to establish
exposure limits for nanosized titanium
dioxide particles.27 This work provides
a framework for developing nanoparticles
and nanomaterial industrial hygiene
procedures. Ongoing nanotoxicity
research is focusing upon the infl uence
of physical and chemical properties
on nanoparticles; short- and long-term
effects on organ systems and tissues;
biological mechanisms for potential
toxic effects; the creation of models
to assist with hazard identifi cation; and
toxicity correlations to mass or other
appropriate measurable nanoparticle
characteristics. 21,22
In the end, nanomaterials present
daunting tasks for researchers and industry,
but also for those who control
limited funds for EH&S activities. Coordination
amongst funding agencies,
such as those proposed by Vicki Colvin,
executive director of the International
Council on Nanotechnology
(ICON),9 is recommended. Collaboration
amongst members of the technical
community is needed to develop predictive
models for exposure risk. Key
factors affecting worker exposure need
to be quantified as do correlations between
exposure potential and work
processes. Also, dose-response relationships
must continue to be developed
with a critical focus on determining
whether a given exposure will lead
to bioaccumulation.8,21,22
The environmental impact of nanoparticles
and nanomaterials is another
important area for continuing research.
Again, characterization and monitoring
studies are needed to construct meaningful
models. Critical questions facing
researchers are:
- Are nanomaterials more toxic
than their non-nano counterparts?
- What is the fate of nanoparticles
in air, water, and soil?
- What is the biodegradation potential
of nanoparticles?
- Will nanoparticles transform in
the environment into a more toxic
form? 16
Similar to human exposure studies,
much work is needed to assess the environmental
impact of nanoparticles.
The effects of nanoparticles on environmentally
relevant species need to be
quantified. The entire food chain must
be considered, ranging from bottom-dwelling
species (benthic) to flora and
fauna to large wildlife.
Karns and Matthews18,28 recommend
paying particular attention to the beginning
and end of the nanomaterials
life cycle, focusing on nanoparticle
emissions and product disposal. Using
screening tools, such as the U.S.
Environmental Protection Agency’s
Tool for the Reduction and Assessment
of Chemical and Other Environmental
Impacts29 and life-cycles analyses, an
assessment of risks and benefits can
be made. In doing so, it is hoped that
nanoparticle applications can be prioritized
for in-depth analyses.
WORKER SAFETY AND
ENVIRONMENTAL STEWARDSHIP
Consensus standards are currently
being investigated by ANSI30 and
ASTM.31 In the absence of those standards,
accepted EH&S anticipation,
recognition, evaluation, and control
processes are being used to ensure
worker safety and control environmental
impact. The following builds upon
this framework and discussions during
the Environmental, Health and Safety
Forum at Commercialization of Nano-
Materials 2007.
Anticipate and Recognize
Material Safety and Data Sheets
(MSDSs) are not readily available for
nanomaterials. Even if an MSDS does
exist for a given chemical or compound,
it most likely is not applicable
for nanoscale particles.
Risk assessments for respiratory,
dermal, or other exposures are needed
prior to initiating nanoparticle research
and nanomaterial production. Past experience
with other nanoparticles, including
those found in nature, should
be considered during this process.
Nanoparticles may represent fire and
explosion concerns. The physical and
chemical properties of the nanomaterials
must be reviewed and procedures
developed cognizant of nanoparticle
combustibility, flammability, and conductivity.
Handling procedures for all
processes, products, and waste forms
need to be implemented. Furthermore,
the nature of nanoparticles and the
disposition of nanomaterials are to be
properly documented.
Communication is a key part of this
process. All workers coming into contact
with nanoparticles must be trained
regarding potential hazards. Government
agencies and academia must
widely circulate data, models, standards,
and regulations in a timely fashion.
Materials and industrial hygiene
professionals also need to maintain
awareness of these advancements.
Evaluate
Work environment monitoring is recommended.
These efforts can involve
direct-read measurements, air sampling,
and passive measurements.15,17
The collected data can establish exposure
potentials, verify effectiveness
of engineering controls, and help
establish dose-response knowledge.
Nanoparticle characterization should
include form, shape, chemical composition,
and size distribution within a
sampling.
Control
Best practices for nanomaterials
EH&S have been released by various
organizations.32–39 Additionally, the
following were recommended during
the Environmental, Health and Safety
Forum at Commercialization of
NanoMaterials 2007:15–17,19
- An EH&S audit should be conducted
prior to beginning nanoparticle
activities.
- Written procedures must document
worker exposure potential,
required engineering controls and
PPE, methods for safe storage and
handling, procedures to minimize
exposure during production or
analysis, waste disposal protocols,
and spill containment practices.
- High-efficiency particulate air filtered
vacuum systems should be
used. Laminar flow filtered laboratory
hoods and negative pressure
around equipment can reduce
worker exposure potential. Also,
filtration on vacuum pumps for
equipment can minimize air emissions
and contamination.
- Personal respirators are not recommended
until ongoing studies
on seal effectiveness are completed.
- Personal protective clothing should
be worn at all times to minimize
skin contact.
- Nanoparticles in liquid or dry particulate
form should be treated as
hazardous or special waste. In the
absence of consensus standards,
best practices for asbestos disposal
should be used for insoluble
nanoparticles.
CONCLUSIONS
Currently, nanomaterials EH&S
knowledge lags commercial development
of them. Coordination among
the technical community and funding
agencies is needed to bridge knowledge
gaps, ensure worker safety, and promote
environmental stewardship. While
nanotoxicity studies proceed, sound
EH&S practices are needed for ongoing
research, development, and commercialization
programs. Companies,
academic institutions, and government
agencies engaged in the nanomaterials
enterprise must anticipate the need
for sound technical data for developing
dose-response behavior and exposure
limits for nanoparticles. They must also
recognize that prioritization and coordination
of efforts is needed to make
meaningful progress. Nanoparticle exposure
levels must be evaluated in laboratories
and production facilities using
effective monitoring techniques. Finally,
best practices must be disseminated
and adopted to control environmental
impact and ensure worker safety.
ACKNOWLEDGEMENTS
Kim McDonald from Bayer MaterialScience
LLC is gratefully acknowledged
for organizing the Environmental,
Health and Safety Forum at Commercialization
of NanoMaterials 2007.
Randy Ogle from Oak Ridge National
Laboratory, Elizabeth McMeekin from
PPG Industries, Inc., Keith Rickabaugh
from RJLee Group, Inc., H. Scott Matthews
from Carnegie Mellon University,
and Ron Shaffer from NIOSH are
also recognized for their presentations
and participation in the panel discussion.
REFERENCES
- K. Zappas, “From Local to International: A Look at Nanotechnology Initiatives,” JOM, 59 (12) (2007), p. 72.
- “The National Nanotechnology Initiative Research: Research and Development Leading to a Revolution in Technology and Industry,” Supplement to the President’s FY 2008 Budget (Washington, D.C.: National Science and Technology Council), 2007.
- G.M. Holdridge, “National Nanotechnology Initiative: Overview and Commercialization Efforts” (Presentation at the Commercialization of NanoMaterials 2007, Pittsburgh, PA, 12 November 2007).
- C. Rohrer and T.M. Osman, “Nanotechnology in Materials Science and Engineering Education,” Materials Technology@TMS (November 2007).
- T.M. Osman et al., “Commercialization of Nanomaterials: Today and Tomorrow,” JOM, 58 (4) (2006), pp. 21–24.
- “Conclusions and Recommendations”, Nanotechnology – Toxicological Issues and Environmental Safety, NATO Science for Peace and Security Series – C: Environmental Security, ed. P.P. Simeonva, N. Opopol and M.I. Luster (New York: Springer, 2007), pp. xi-xv.
- A.D. Maynard, “Nanotechnologies: Overview and Issues,” Nanotechnology – Toxicological Issues and Environmental Safety, NATO Science for Peace and Security Series – C: Environmental Security, ed. P.P. Simeonva, N. Opopol, and M.I. Luster (New York: Springer, 2007), pp. 1-14.
- “Environmental, Health and Safety Research Needs for Engineered Nanoscale Materials” (Washington, D.C.: National Nanotechnology Coordination Office, August 2006).
- V.L. Colvin, “Research on Environmental and Safety Impacts of Nanotechnology: Current Status of Planning and Implementation under the National Nanotechnology Initiative,” Testimony to the United States House of Representatives Committee on Science and Technology, Technology (31 October 2007).
- “Nanotechnology Recent Developments, Risks and Opportunities,” Lloyd’s Emerging Risk Team Report (London: Lloyds, 2007).
- S. Wood, R. Jones, and A. Geldart, “The Social and Economic Challenges of Nanotechnology” (Swindon, U.K.: Economic and Social Research Council, 2005).
- P.A. Schulte and F. Salamanca-Buentello, “Ethical and Scientific Issues of Nanotechnology in the Workplace,” Environ Health Perspect., 115 (January 2007), pp. 5–12.
- A. Maynard, “Is Engineered Nanomaterial Exposure a Myth?” (Safenano, U.K.: 2 October 2007).
- K.R. McDonald, “Nanomaterials: An HSE Overview” (Presentation at the Commercialization of NanoMaterials 2007, Pittsburgh, PA, 12 November 2007).
- R. Ogle, “Application of Industrial Hygiene Tools and Tenets to Controlling Nanomaterials in R&D Operations” (Presentation at the Commercialization of NanoMaterials 2007, Pittsburgh, PA, 12 November 2007).
- E. McMeekin, “Environmental and IH Considerations in Nanomaterial Production and Use” (Presentation at the Commercialization of NanoMaterials 2007, Pittsburgh, PA, 12 November 2007).
- K. Rickabaugh, “Laboratory Workplace Safety Practices and Sampling and Analysis Considerations” (Presentation at the Commercialization of NanoMaterials 2007, Pittsburgh, PA, 12 November 2007).
- H.S. Matthews, “Life Cycle Impacts of Nanotechnology” (Presentation at the Commercialization of NanoMaterials 2007, Pittsburgh, PA, 12 November 2007).
- R. Shaffer, “An Overview of NIOSH Nanotechnology Research and an Update on the Efficacy of Personal Protective Equipment for Reducing Worker Exposure to Nanoparticles” (Presentation at the Commercialization of NanoMaterials 2007, Pittsburgh, PA, 12 November 2007).
- “Final Nanotechnology White Paper” (Washington, D.C.: United States Environmental Protection Agency, 15 February 2007).
- C. Geraci, “The NIOSH Nanotechnology Research Program” (Presentation at Commercialization of NanoMaterials 2006, 20 September 2006).
- “Critical Topic Areas” (Washington D.C., 2007: NIOSH).
- “FDA Nanotechnology Task Force Report” (Washington, D.C.: United States Food and Drug Administration, 1 July 2007).
- C.M. Garner, “ICON NanoEHS Research Roadmap Proposal,” (Houston, TX: International Council on Nanotechnology, 10 May 2006).
- P.D. Zeigler, “Nantechnology: Managing EH&S Issues and Regulations with an Emerging Technology” (Presentation at Commercialization of NanoMaterials 2006, 20 September 2006).
- “Recommendations from the Toxicology Working Group of the Nanotechnology Panel of the American Chemistry Council for a Reasoned Approach to the Testing of Nanoscale Materials, (Arlington, VA: American Chemistry Council, 2006).
- “Evaluation of the Health Hazard and Recommendation for Occupational Exposure to Titanium Dioxide, NIOSH Current Intelligence Bulletin (Washington, D.C.: NIOSH, 22 November 2005).
- B. Karn and H.S. Matthews, “Nanoparticles without Macroproblems,” IEEE Spectrum, (September 2007), pp. 55–58.
- “Tool for the Reduction and Assessment of Chemical and Other Environmental Impact (TRACI)” (Washington, D.C.: United States Environmental Protection Agency, 2007).
- “ANSI Nanotechnology Standards Panel” (New York: ANSI, 2007).
- “Committee E56 on Nanotechnology” (West Conshohocken, PA: ASTM, 2007).
- “Approach to Nanomaterials ES&H,” Revision 2 (United States Department of Energy Nanoscience Research Centers, June 2007.
- “Approaches to Safe Nanotechnology: An Information Exchange with NIOSH,” Version 2.0 (Washington, D.C.: NIOSH, 2006).
- “Progress Toward Safe Nanotechnology in the Workforce,” Publication 2007-123 (Washington, D.C.: NIOSH, 2007).
- “Prudent Practices in the Laboratory Handling and Disposal of Chemicals (Washington, D.C.: National Research Council, National Academies Press, 1995).
- “Nanotechnology Consensus Workplace Safety Guidelines (Washington, D.C.: ORC Worldwide, 2005).
- “A Survey of Current Best Practices in Nanotechnology (Houston, TX: International Council on Nanotechnology, 13 November 2006).
- “Current Knowledge and Practices Regarding Health and Safety in the Nanotechnology Workplace (Houston, TX: International Council on Nanotechnology, 18 October 2006).
- “Nano Risk Framework,” Environmental Defense-DuPont Nano Partnership, June 2007.
Todd M. Osman is Technical Director at TMS, 184
Thorn Hill Road, Warrendale, PA 15086; e-mail
tosman@tms.org.
|