LATEST ISSUE |
||||
TMS QUICK LINKS: |
• TECHNICAL QUESTIONS • NEWS ROOM • ABOUT TMS • SITE MAP • CONTACT US |
JOM QUICK LINKS: |
• COVER GALLERY • CLASSIFIED ADS • SUBJECT INDEXES • AUTHORS KIT • ADVERTISE |
|
| Corrosion: Overview | Vol. 62, No.6 pp. 32-43 |
Transformation of Micro-to-Macroscopic 3-D Structures
Kenneth H. Sandhage
Questions? Contact jom@tms.org. |
|
The scalable fabrication of nanostructured materials with complex morphologies and tailorable chemistries remains a significant challenge. One strategy for such synthesis consists of the generation of a solid structure with a desired morphology (a "preform"), followed by reactive conversion of the preform into a new chemistry. Several gas/solid and liquid/solid reaction processes that are capable of such chemical conversion into new micro-to-nanostructured materials, while preserving the macroscopic-to-microscopic preform morphologies, are described in this overview. Such shape-preserving chemical transformation of one material into another could be considered a modern type of materials "alchemy." Scalable fabrication protocols for the syntheses of structures with functional chemistries and complex morphologies that can be tailored over various length scales (even down to the nanoscale) may have a significant impact in a variety of current or potential applications. One paradigm for such fabrication involves separation of the processes for structure formation and for chemical tailoring; that is, a solid structure (a "preform") of a given chemistry may first be fabricated with desired macro-to-nanoscale morphological features, and then converted into a new chemistry via morphology-preserving gas/solid or liquid/solid reaction(s). The extensive literature available on the kinetics and phase evolution associated with such reactions in the field of high temperature oxidation/corrosion provides a rich source of mechanistic information that can be utilized for such materials "alchemy."
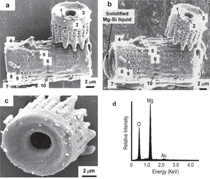
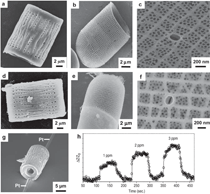
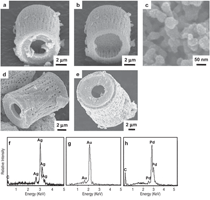
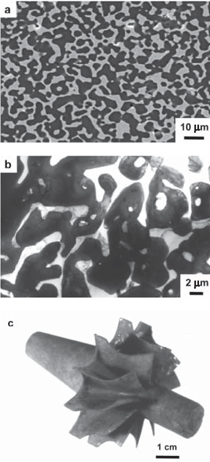
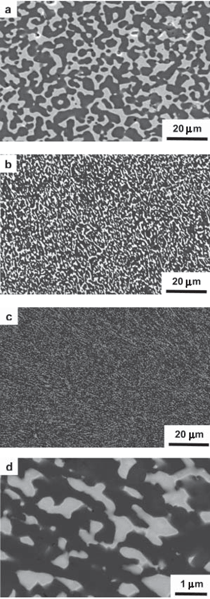
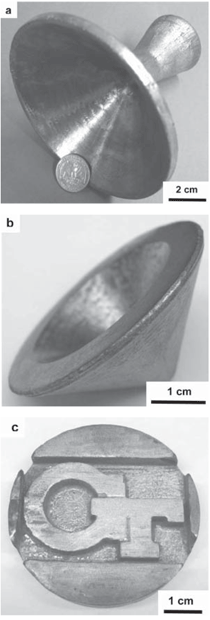
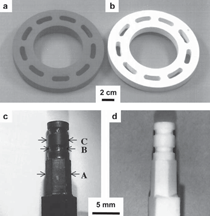
The purpose of this overview is to provide examples of the following three reaction-based approaches for the chemical conversion of shaped solid preforms: gas/solid displacement reactions, liquid/solid displacement reactions, and gas/solid oxidation reactions. Under appropriate conditions, each of these reaction-based approaches can be used to transform solid preforms into new nanostructured materials while preserving the preform morphology (note: a "nanostructured material" refers herein to a material with one or more physical features, such as a phasesize, crystal size, and/or pore size, with a dimension below one micrometer). The syntheses of two types of shaped, nanostructured materials will be discussed here: microscopic structures with nanoscale features (generated via the application of gas/solid displacement reactions to solid microscopic preforms), and macroscopic structures with nanoscale phase and/or crystal sizes (generated via the application of liquid/solid displacement reactions, or gas/solid oxidation reactions, to solid macroscopic preforms). Biologically Replicable, 3-D, Microscale, Nanostructured Preforms: Diatom Frustules Oxide, Oxide/Metal, and Metal Replicas via Oxidationreduction Displacement Reactions The gas/solid magnesiothermic reaction of an oxide template may also be used to generate a nanocrystalline elemental replica of the template. By lowering the magnesiothermic reaction temperature to 650°C, and by reducing the molar Mg:SiO2 reactant ratio to a value just above that required by the stoichiometry of Reaction 1, the formation of an Mg2Si compound and the Mg-Si alloy liquid were suppressed, so that nanocrystalline MgO/Si composite replicas of diatom frustules were formed, as indicated by Reaction 2.17–21 The products of this reaction correspond to a co-continuous, nanocrystalline mixture of MgO (65.1 vol.%) and Si (34.9 vol.%). Owing to the continuity of the Si phase, porous Si replicas of the diatom frustules have been produced by selective acid dissolution of the interconnected MgO network.21 As revealed in Figure 2, the 3-D frustule morphology and features as small several tens of nanometers were well-preserved in the resulting nanocrystalline Si replicas. The Si frustule replicas possessed a much higher specific surface area (>500 m2/g) than the starting SiO2 frustules,and contained a high population of nanoscale (<2 nm diameter) pores.21 Such a single porous silicon frustule replica was found to act as a rapid, sensitive, low-voltage, and minimally invasive gas sensor (Figures 2g and h).21 This now-patented8,9,22,23 shape-preserving magnesiothermic reduction process has also recently been used to convert synthetic mesoporous silica films and colloidal silica assemblies into porous silicon replicas.24–26 Such nanocrystalline porous silicon replica structures, generated from biosilica or synthetic silica templates, can be attractive for a number of chemical, biochemical, electrical, and optical applications (e.g., for sensing, biocatalysis, batteries, and photovoltaics).21,24–29 Szczech and Jin30 have subsequently reported that Mg2Si-bearing diatom frustule replicas generated by this magnesiothermic conversion process may be useful for thermoelectric applications (note: the formation of such solid Mg2Si, relative to solid Si or Mg-Si liquid products, may be controlled by adjustment of the Mg:SiO2 reactant ratio at modest temperatures20,21). Oxide Replicas via Metathetic (Halide) Displacement Reactions Metathetic gas/solid displacement reactions, involving halide gases, have also been utilized for the shape-preserving conversion of intricate 3-D silica microparticles, ordered porous silica films, and silica microspheres into nanocrystalline replicas comprised of other oxides.31–36 For example, the exposure of diatom SiO2 frustules to TiF4 gas, generated by the vaporization of solid TiF4, has been used to transform the frustules into titanium oxyfluoride, TiOF2(s), according to a metathetic displacement reaction (Reaction 3).31,32 Initial experiments conducted at 500–700°C with molar TiF4:SiO2 reactant ratios >4.9:1 resulted in reactive evaporation and disintegration of the silica frustules (Figures 3a–c).31,32 Such reactive silica evaporation (a type of active corrosion37,38) at such modest temperatures indicated that a volatile Si-O-bearing gas species had formed. Hexafluorodisiloxane gas, Si2OF6(g), is one such likely species that can form by Reaction 4. The apparent formation of Si2OF6(g), and the associated vaporization of the SiO2 template, was avoided by conducting the metathetic reactive conversion at lower temperatures (i.e., 180–350°C) and at lower molar TiF4:SiO2 reactant ratios (<2.4:1).31–34 Under these conditions, the SiO2 reactive evaporation was suppressed and the TiOF2 product formed on the SiO2 frustule surfaces (i.e., analogous to a transition from active to passive corrosion37,38). Complete reaction then yielded nanocrystalline TiOF2 structures that retained the SiO2 frustule shape and fine features.31–33 Such TiOF2 replicas were then converted into nanocrystalline anatase TiO2-based replicas (Figures 3d and e) via reaction with humid oxygen at ≥600°C as shown in Reactions 5a and 5b,32,33 where TiO2–aF2a(s) refers to fluorine-doped anatase titania. The open nanocrystalline nature of these anatase microparticles, along with the retention of a controlled amount of fluorine (via tailoring of the humid oxygen treatment), enabled these doped titania replicas to act as effective agents for the rapid hydrolytic destruction of pesticides (methyl paraoxon and methyl parathion) in water without the need for UV light.33 This patented shape-preserving metathetic reaction process8,9,22 has also been used to convert opalescent 3-D porous silica preforms into nanocrystalline titania structures that retained the general morphology and structural features of the preforms.35 Given the chemical, biochemical, optical, and electrical properties of titania, the ability to convert 3-D silica microparticles, ordered opalescent films, microspheres, or other silica-derived morphologies into nanocrystalline titania replicas can be quite attractive for generating sensitive, minimally-invasive gas detectors (e.g., for CO(g) or H2(g)), efficient photocatalysts (e.g., for the reaction of detergents or dyes in water), indextailored waveguides (e.g., for visible or near IR wavelengths), controlled-shape pigment particles (e.g., for paints, paper, plastics, ink, and cosmetics), nanostructured medical implants (e.g., as biocompatible coatings for bone implants), efficient antimicrobial agents (e.g., for killing E. coli bacteria), and highly-porous electrodes (e.g., for dyesensitized solar cells).39–44Further Chemical Modification of Replicas via Coating and/or Additive Reactions The new oxide chemistries of replica structures generated by gas/solid displacement reactions can enable the coating of these replicas with additional functional oxides (i.e., so as to yield multilayered and/or multicomponent replicas). Consider, for example, the syntheses of BaTiO3-based coatings on shaped microtemplates (e.g., intricate microparticles, porous inverse opals, or patterned films). Such templated Ba-TiO3-based structures can be attractive for electronic (e.g., capacitor, varistor), electrochemical (e.g., gas sensing), thermal (e.g., temperature sensing), or optical (e.g., phosphor) applications.45–47 However, BaTiO3 is not thermochemically compatible with SiO2; that is, the deposition of a Ba-Ti-O-bearing coating on a SiO2 template, followed by thermal treatment to allow for conversion of the coating into crystalline BaTiO3, tends to result in the formation of undesired silicate compounds (e.g., BaTiSiO5, BaTiSi2O7, BaTiSi3O948). Fortunately, MgO replicas of SiO2 templates are chemically compatible with BaTiO3.49 Furthermore, because nanocrystalline magnesia surfaces can be readily hydroxylated, magnesia templates are attractive for reaction with, and binding of, alkoxide precursors used in sol-gel deposition processes. Weatherspoon et al.50–52 have demonstrated the efficacy of such a combined magnesiothermic reaction and sol-gel coating approach by generating BaTiO3 coatings on MgO diatom frustule replicas. After magnesiothermic conversion of silica frustules at 900°C for 1.5 h, the magnesia frustule replicas were immersed in a heated aqueous NaOH solution. The resulting hydroxylated magnesia replicas were then exposed to a refluxed solution of barium titanium ethylhexanoisopropoxide in ethanol at 70°C. After evaporation of the volatile components of the solution at 56°C, the coated frustules were heated in air to 700°C for 1.5 h to allow for organic pyrolysis and crystallization of the coating. The resulting frustules contained a thin (150 nm), continuous, and conformal coating of nanocrystalline BaTiO3 (average crystal size of 20 nm). Indeed, the coating was sufficiently continuous as to yield freestanding BaTiO3 frustule replicas upon selective dissolution of the underlying MgO template.52 A similar react-and-coat process has also used by these authors to synthesize photoluminescent Eu-doped BaTiO3- bearing frustule replicas.51Nanostructured elemental replicas of shaped oxide templates, generated through a magnesiothermic reduction (displacement reaction) process, may also be used as templates for subsequent electroless deposition of other functional elements. The coating of relatively noble metals (Au, Ag, Pd) on porous nanostructured templates can be attractive for a number of catalytic (e.g., for CO(g) oxidation, fuel cell catalysts), electrochemical (e.g., sensors), electronic (e.g., electrodes), thermal (e.g., cryogenic heat exchangers), and other applications.53–55 While the direct electroless deposition of noble metal coatings on silica templates is inhibited by the insulating nature of this oxide, porous silicon replicas possess appropriate electronic and chemical (reducing) characteristics for such deposition. Bao et al.56 have recently synthesized nanocrystalline noble metal replicas of 3-D silica microparticle templates (diatom frustules) by first converting the silica into porous silicon (via magnesiothermic reduction), and then applying metal coatings onto/within the porous silicon via subsequent electroless deposition. Subsequent selective dissolution of the Si in an aqueous NaOH solution then yielded freestanding porous Ag, Au, or Pd structures that retained the 3-D morphology of the starting silicon frustule replicas (Figure 4).56 The frustule-shaped silver, gold, and palladium microparticles possessed average crystal sizes of only 14, 50, and 43 nm, respectively. Oxide replicas of patterned templates, generated via metathetic gas/solid displacement reactions, can also be used as reactive templates for the generation of functional multicomponent oxides. For example, nanocrystalline titania replicas produced by the reaction of silica templates with titanium tetrafluoride gas (using Reactions 3, 5a, and 5b) have been converted into barium titanate (BaTiO3) replicas through hydrothermal reaction by Ernst, et al.52,57 The titania replicas were sealed, along with barium hydroxide octahydrate and water, inside a Teflon chamber and then heated to 100°C for 48 h to allow for hydrothermal conversion into barium titanate. The resulting frustule-shaped particles were found to be comprised of nanocrystalline BaTiO3 with an average crystal size of only 63 nm.52,57 Several liquid/solid displacement reaction- based approaches have been developed to allow for the conversion of shaped, macroscopic, ceramic-bearing preforms into composites comprised of new ceramic and metallic or intermetallic products that retain the shapes and dimensions (to within 1%) of the starting preforms. Under appropriate conditions, these approaches have yielded composites with micrometer-to-submicrometer phase and/or crystal sizes. Two general categories of such liquid/ solid displacement reaction-based processes are reactive metal penetration and reactive casting. Reactive metal penetration refers to the conversion of a dense, shaped ceramic-bearing preform into a dense product, comprised of new ceramic and metallic or intermetallic phases, via the inward migration of a liquid/solid reaction front through the dense preform.58–69 Reactive casting refers to the conversion of a porous, shaped ceramic-bearing preform into a dense ceramic/metallic or ceramic/ intermetallic composite via the infiltration (with or without the assistance of an applied pressure) of a metallic liquid through the porous preform and reaction of this liquid with one or more solid phases in the preform.70–85 Reactive Metal Penetration A common displacement reaction used in reactive metal penetration is shown in Reaction 6,58–60 where {Si} refers to silicon dissolved within an aluminum- silicon melt. With this process, dense amorphous SiO2 preforms, which may be readily fabricated into complicated shapes through conventional glass forming methods, are immersed in a bath of molten Al at ≥1,000°C. The reaction of a silica preform with molten aluminum (Reaction 6) proceeds by the formation of an Al2O3-bearing reaction zone that migrates through the preform at a linear rate (on the order of 1–5 mm/ h) at 1,000–1,200°C.59 The volume of 2 moles of Al2O3(s) is substantially less than the volume of 3 moles of SiO2(s). Hence, this reaction-induced volume reduction generates space that is accommodated by the molten metal. The silicon product of this displacement reaction can dissolve into the molten metal and then diffuse into the excess surrounding aluminum bath at ≥1,000°C.59 Upon cool down, the resulting composites are comprised of a fine mixture of interpenetrating Al2O3 and Al-rich phases (Figures 5a and b). (Note: owing to the continuity of both phases, this reactive metal penetration process has also been referred to as the co-continuous ceramic composite, or C4, process by Breslin, Daehn, and colleagues, who pioneered this approach. 58–61) An interesting microstructural characteristic of such composites is the growth texture of the alumina product phase; that is, the c-axis of the α-Al2O3 phase is strongly aligned with the direction of migration of the reaction front.61 This reactive metal penetration process yields co-continuous Al2O3/Al-rich composites that retain the shape and dimensions (to within 1%) of the starting dense SiO2 preforms (Figure 5c).58–61 Reactive metal penetration has also been used to convert dense aluminosilicate (e.g., mullite, Al6Si2O13; sillimanite, Al2SiO5) preforms into dense, near net-shape, co-continuous composites of Al2O3 and Al-rich alloy (or Al-rich alloy + Si).62–64 The values of specific modulus (62–86 GPa·cm3/g), hardness (8–12 GPa), toughness (5–10 MPa·m1/2), and thermal conductivity (80 WK–1m–1), and the wear behavior reported for these lightweight (3.4–3.7 g/cm3) Al2O3/Al-bearing composites make such reaction-formed materials attractive for applications such as automotive disk brake rotors and calipers, internal combustion engine piston crowns, turbine compressors, and cylinder liners.58–62,65 The thermal and mechanical performance of such cocontinuous composites have been further tailored through modifications of the preform and melt chemistries. For example, the reaction of shaped preforms comprised of mixtures of SiC and SiO2 with an Al-Si alloy melt has yielded SiC/Al2O3/Al-based composites with enhanced thermal conductivity, thermal shock resistance, and wear resistance.60,65 The continuous metallic phase has also been modified by immersing shaped Al2O3/Al-bearing composites in a second bath of another metal alloy to allow for chemical alteration via liquid phase diffusion and exchange. For example, such a liquid exchange process has been used to generate composites with continuous Cu-Al-Fe, Fe-Al, or Ni-Al phases for higher temperature applications.60,61,66The average size of the oxide and metal colonies within co-continuous alumina/aluminum-bearing composites formed by reactive metal penetration of silica-bearing or mullite-bearing preforms at 1,000–1,200°C is typically several micrometers, although a significant population of submicrometer sized ligaments has also been observed.59,64,67 The oxide and metal colony size can be appreciably reduced, however, through alloying additions to the molten aluminum. For example, Strange and Breslin,68 and Evarts69 have reported that the introduction of copper into the aluminum melt has a dramatic impact on the scale of the microstructure of composites formed by reactive metal penetration. These authors immersed dense amorphous SiO2 preforms into Cu-Al melts comprised of 30–63 at.% (50–80 wt.%) Cu at 1,100–1,150°C. The rates of linear penetration for these copper-aluminum alloy melts into the silica preforms were lower than for pure molten aluminum. Dramatic reductions in the colony sizes of the co-continuous oxide and intermetallic (AlCu, Al2Cu) phases were observed for melts with ≥50 at.% Cu (Figures 6a–d). The nanostructured Al2O3/AlCu/ Al2Cu composites were also found to exhibit substantially higher values of hardness than microstructured Al2O3/ AlCu/Al2Cu composites.69 Yoshikawa et al.66 have reported that the reactive metal penetration of molten Fe-Al alloys into dense amorphous SiO2 rods at 1,200–1,300°C yielded co-continuous, nanocrystalline Al2O3/FexAly composites. 66 With increasing iron content in the melt (from 5–30 at.% Fe), the rate of reactive metal penetration into the silica preform decreased and composites with iron-aluminum intermetallic phases of higher iron content were generated (i.e., predominantly Al3Fe for a 20 at.% Fe melt vs. predominantly Al5Fe2 for a 30 at.% Fe melt).66 Reactive Casting Reactive casting of molten metals or alloys into porous ceramic-bearing preforms has also been used to generate near net-shaped composites comprised of new ceramic (e.g., Al2O3, MgO, MgAl2O4, ZrC, HfC) and metallic (e.g., Al, Mg-Al, Fe-Ni-Al, Fe-Ni-Cr, Fe-Cr- Al, Ni-Co-Cr-Al, W) or intermetallic (e.g., NbAl3, Nb2Al, TiAl3, Ti(Al,Si)3, NiAl, Ni3Al, FeAl) phases.58,60,70–85 Prior to such reactive casting, a reactant- bearing powder or powder mixture, containing appropriate ceramic or ceramic and metal constituents, is first shaped into a rigid preform of desired morphology and porosity. Preforms of complex morphology have been prepared by a variety of methods, including slip or gel casting, green machining, powder injection molding, or rapid prototyping approaches.79,80,85 Some firing of the preform is typically conducted prior to pressureless reactive infiltration to allow for some particle necking in the preform (for sufficient rigidity to avoid shape distortion during reactive casting) and to obtain a desired level of preform porosity. Indeed, the final phase content of composites generated by reactive casting may be tailored by adjusting the porosity, as well as the phase content, of the shaped rigid preform. Consider, for example, the net liquid/solid displacement Reactions 7a, 7b, 8, and 9,70–80 where {Al}, {Mg}, and {Zr} refer to aluminum, magnesium, and zirconium present within a melt. Like for Reaction 6, displacement Reactions 7a and 7b generate oxide products that possess a smaller volume than the oxide reactant (e.g., the volume of 2 moles of α-Al2O3 is 9.3% smaller than the volume of 3 moles of rutile TiO286). Such a reaction-induced reduction in the internal solid oxide volume, coupled with the starting open pore volume of the rigid preform, provides the space required to accommodate the formation of the solid titanium aluminide product (via the reactive casting approach known as the infiltration alumina aluminide alloy, or i-3A, process70–72). By tailoring the starting preform porosity, the aluminum content of the aluminide phase (e.g., TiAl3 vs. TiAl) and the relative amount of aluminide in the final dense composite may be adjusted.70–72 Displacement Reactions 8 and 9, on the other hand, generate more ceramic volume than is consumed (e.g., the volume of 3 moles of MgO is 32% larger than the volume of 1 mole of α-Al2O3). Such a reaction-induced increase in the internal solid volume within a reacting, rigid preform can be used to generate ceramic/metal or ceramic/intermetallic composites with relatively high ceramic contents (via the reactive casting approach known as the displacive compensation of porosity, or DCP, process73– 80). Indeed, electrically-insulating MgO/Mg-Al composites comprised of ≥83 vol.% MgO have been produced by such a reactive casting process; that is, sufficient metallic liquid was extruded out of the rigid specimen during reactive conversion of Al2O3 into MgO (Reaction 8) that the remaining entrapped metallic (Al-Mg) phase was discontinuous.73 Because the partially sintered (necked) preforms remain rigid during such reactive infiltration, porous preforms of complex shape, fabricated by gel casting,79 green machining,80 or three-dimensional printing,80 have been converted into dense ceramic-rich products that retain the shapes and dimensions (to within 1%) of the starting porous preforms (Figure 7a–c).The sizes of ceramic and metal or intermetallic phases generated within reactively cast composites can be appreciably refined through the use of fine-scale reactant phases in the starting porous preforms, rapid molten metal infiltration at a modest temperature, and modest post-infiltration heat treatment.71,72,82 For example, Al2O3/ Ti(Al,Si)3-based composites with phase sizes of a few micrometers to submicrometers have been produced by: i) attrition milling of a mixture of fine Al2O3 (1.2 μm ave. size) and TiO2 (0.5 μm ave. size) powders, ii) compacting and sintering the powder mixture for 0.5 h at 1,150°C (to generate a rigid preform with a porosity of 49 vol.%), iii) rapid squeeze casting (within a few seconds) of a molten Al-Si alloy at 700°C into the porous preform, and then iv) annealing of the resulting infiltrated preform for 3 h at 800°C.72,82 While the as-cast specimen contained unreacted TiO2, Al, and Si phases and no apparent Ti(Al,Si)3 product (as determined from x-ray diffraction analysis), the subsequent 800°C/3 h thermal treatment resulted in complete conversion of the as-cast specimen into an intimate mixture of Al2O3 and Ti(Al,Si)3. Such fine-scale, reactively cast, near net-shape Al2O3/Ti(Al,Si)3 composites possessed fracture strengths (4 point bending) of 490–540 MPa, fracture toughness (by indentation) values of 5.0–8.6 MPa·m1/2, and hardness values of 5.7–7.4 GPa.72,82 A similar approach (milling of fine oxide/metal powders, partial sintering, rapid squeeze casting, and modest annealing after casting) has been used to fabricate near net-shaped metal matrix composites (Fe-Ni-Cr-based or Ni-Co-Cr-Al-based) reinforced with nanoscale Al2O3 filaments (via the reactive casting approach known as the infiltration Metal Matrix Composite process81–85). The fracture strengths of reactively cast Fe-Ni-Crbased alloy composites reinforced with nanoscale Al2O3 at 550°C and 900°C were found to be almost twice the values of a similar reference metal alloy lacking the alumina phase.83 A derivative of such reactive casting ("in-situ" or "short-distance" infiltration82,85,87,88), involving local aluminum melting and brief reaction within attrition-milled oxide/metal mixtures under an applied pressure, has also been used to synthesize dense metal alloy matrix composites reinforced with interconnected Al2O3 filaments of a few hundred nanometers thickness. Such Fe-Cr-Ni alloy/ nanofi lamentary Al2O3 composites have exhibited fracture strength and fracture toughness values of 1,100 MPa and 18 MPa·m1/2, respectively, in threepoint bending.88 Two general oxidation-based approaches that are capable of converting shaped, macroscopic, freestanding, solid metal-bearing preforms into oxide products that retain the preform shape with relatively little or no change in dimensions (Figure 8) are the reaction bonded metal oxide (RBMO) process, and the volume identical metal oxidation (VIMOX) process. (Note: while the reaction bonded metal oxide, RBMO, process is often referred to as the reaction bonded aluminum oxide, or RBAO, process, in light of the pioneering work of Claussen et al.,89,93–100 the RBMO label is used herein, as this process may be used to generate a variety of metal oxides.) Use of Oxidation-induced Volume Changes for Near Net-Shape Processing For both of the patented RBMO89 and VIMOX processes,90,91 the volume change(s) associated with oxidation are used to partially or fully offset opposing volume changes resulting from other phenomena. Consider the oxide-to-metal volume ratios for several elements, often referred to as the Pilling Bedworth Ratio (PBR), presented in Table II.92 For the elements in the left half of Table II, the PBR values are well in excess of unity (as is true for most elements). For a solid, porous preform containing one or more of these elements (as well as perhaps other constituents that exhibit an increase in solid volume upon oxidation, such as silicon carbide), the oxidation-induced increase in solid volume may be offset by the sintering-induced shrinkage during post-oxidation annealing. Hence, a shaped porous preform containing tailored amounts of metal, ceramic, and porosity may be converted, with appropriate oxidation and sintering treatments, into a dense all-oxide body that preserves the preform shape with relatively little or no net shrinkage. For example, porous green-machined bodies of Al, Al2O3, SiC, and ZrO2 have been converted into dense composites of Al6Si2O13 (mullite) and ZrO2 that retain the preform shape and dimensions (to within 1%).93 This is the basic premise for near net-shape processing by the RBMO method. The RBMO approach has been extensively utilized to generate low-shrinkage Al2O3-based (e.g., Al2O3, Al2O3-ZrO2, Al2O3-ZrO2-Nb2O5) or Al6Si2O13(mullite)-based (Al6Si2O13, Al6Si2O13-ZrO2, Al6Si2O13-SiC, Al6Si2O13-SiC-ZrO2) materials for applications requiring high stiffness, strength, wear resistance, and modest weight (e.g., gears, dies, punches, or dental implants).94–101For the alkali and alkaline earth elements shown in the right half of Table II, the PBR values are well below unity. Such oxidation-induced reductions in solid volume may be used to accommodate volume expansions resulting from the oxidation of other elements and/or the formation of oxide compounds. Hence, a shaped dense preform containing tailored amounts of alkali or alkaline earth elements and other metallic and/or ceramic phases may be converted, by appropriate oxidation and post-oxidation annealing treatments, into a dense all-oxide body that retains the shape and dimensions of the preform. For example, a compacted green body of Ba, Sr, Al, Al2O3, and SiO2 has been converted into a (Ba,Sr)Al2Si2O8 (celsian) body that retained the preform shape and dimensions (to within 1%).102,103 This is the basic premise for near net-shape processing by the VIMOX approach. The VIMOX approach has yielded a variety of near net-shaped, functional, alkaline earth oxide-bearing components (e.g., ferroelectric BaTiO3, PTCR (Ba,Pb)TiO3, proton-conducting BaCeO3, ferrimagnetic BaFe12O19, biocompatible Ca6(PO4)10(OH)2, and refractory MgAl2O4, BaAl2O4, and BaAl2Si2O8).102–115 Fabrication of Shaped, Metal-bearing Preforms The fabrication of solid RBMO and VIMOX preforms typically involves the preparation of a powder mixture comprised of desired metal and ceramic phases, and then compaction and forming of the powder mixture into a green body of desired shape, porosity, and strength. For both approaches, preforms comprised of fine, intimate mixtures of metal and ceramic phases are required in order to obtain desired oxidation and reaction kinetics. High-energy ball milling can be an effective means of preparing such fine, reactive mixtures, although proper care needs to be taken during such milling to avoid undesired reactions (e.g., excessive oxidation and hydration of metallic constituents, formation of brittle intermetallic compounds) and excessive incorporation of wear debris from the milling media and milling vessel into the powder mixture. Proper optimization of milling intensity (e.g., rotation speed during attrition milling), ball-to-charge ratio, starting oxide particle size, milling time, and milling fluid is required to obtain metal/ceramic mixtures of desired composition, phase content, and phase size for subsequent compaction, forming, and oxidation/reaction treatments.94–98,100,103,105,107,116,117 A sufficient amount of ductile metal in the milled powder mixture is required to allow for uniform compaction and forming into a green body of relatively high strength, and to achieve the desired oxidation-induced volume change(s) for shape and dimension preservation. Compacted RBMO and VIMOX powder mixtures with ≥30 vol.% ductile metal can readily be machined, using conventional steel tooling, into intricately-shaped green bodies.93,96,100,103,112,114,118 Metal-rich alkaline-earth-bearing precursors (e.g., with ≥60 vol.% metal) have also been rolled into thin (down to 25 μm) tapes or drawn into multifilamentary (down to 250 nm diameter filaments) wires.103,104,107,108,111,119,120Oxidation Processing The conversion of metal-bearing RBMO and VIMOX green bodies into shape-preserved, oxide-based bodies of desired phase content and microstructure requires the use of controlled oxidation and post-oxidation annealing treatments. Particular concerns associated with the oxidation treatment include: the generation of appreciable metallic liquid due to incomplete oxidation at subsolidus temperatures, and thermal runaway (ignition) associated with the exothermic nature of the oxidation reactions.100,104,105,121,122 Excessive metallic liquid formation (with or without ignition) can lead to undesired agglomeration and phase coarsening, an uncontrolled change in preform composition or porosity (due to the loss of a nonwetting metallic liquid from the preform), and/or a distortion in the preform shape.100,104,108,111 Ignition can lead to the extensive formation of defects (cracks, pores) and geometric distortions due to thermal stresses associated with steep temperature gradients and chemical stresses associated with sharp compositional gradients.121,122 In the RBMO process, the oxidation treatment is typically conducted with a prolonged heat treatment (involving a slow heating rate and/or an extended isothermal anneal) below the melting point of aluminum to allow for extensive subsolidus oxidation.96–98,117 The time required for such subsolidus aluminum oxidation in the RBMO process has been reported to depend upon the amount of aluminum, the aluminum particle size and the preform porosity (which are affected by the milling and compaction conditions), as well as the preform size.98,117,121 In order to dramatically shorten the required oxidation time while avoiding ignition, particularly for green bodies with relatively large characteristic dimensions, feedback control firing (using the rate of specimen weight gain to control heating), has successfully been applied to RBAO green bodies.122 With PBR values less then unity, porous (nonprotective) oxide scales tend to form on alkaline earth metals (Mg, Ca, Sr, Ba) during oxidation at modest temperatures (e.g., at 300–500°C), so that the oxidation of VIMOX preforms can occur at a relatively rapid rate at subsolidus temperatures, even for green bodies of low initial porosity.92,102–115 However, the thermally-insulating nature of the resulting porous alkaline earth oxides requires that a modest rate of heating be used during subsolidus oxidation to avoid ignition and the associated thermal and chemical stresses and defects/distortions.92,103,105,111,114Post-oxidation Annealing The subsolidus oxidation of VIMOX preforms can yield fine nanocrystalline oxide-bearing mixtures that, in turn, can react with other metal or oxide constituents in the preforms to generate binary oxide compounds at low temperatures. For example, the oxidation of barium in air or oxygen at 300°C yields a fine-grained peroxide, BaO2, that reacts rapidly at modest temperatures with other metallic constituents to yield binary oxide compounds (e.g., the reaction of BaO2 with Al, Si, and Ti has yielded BaAl2O4, Ba2SiO4, and Ba2TiO4, respectively, at 300–550°C—well below the melting points of Al, Si, and Ti).103,105,106,109,113,124,125 Continued firing and reaction of such oxidized VIMOX preforms has yielded a variety of fully-reacted, functional multicomponent oxides at modest temperatures. For example, dense tapes of the superconductor, Bi2Sr2Ca1Cu2O8+x, and of the proton conductor, Ba(Ce,Nd)O3, have been produced from oxidized metal-rich precursor tapes after firing at peak temperatures of only 860°C and 900°C, respectively.103,126 Dense composites comprised of alternating layers enriched in fine (submicrometer thick) platelets of superconducting YBa2Cu3O7-y and in Ag have been generated via an oscillating internal oxidation treatment of Y-Ba-Cu-Ag metallic precursor tapes at 400–600°C followed by firing at a peak temperature of 900°C.127 Intentionally porous tapes of nanocrystalline doped (Ba,Pb)TiO3 (a thermistor material with a relatively high Curie temperature) and ferrimagnetic BaFe12O19 have been generated at 750°C and 900–1,080°C, respectively.103,108,111RBMO preforms have also been converted into dense, nanocrystalline oxide-based materials. With proper use of high-energy milling to obtain a fi nely-divided, well-intermixed assemblage of reactive metallic and ceramic phases, with appropriate conditions of compaction and oxidation, and with the use of fine second phases to inhibit grain growth during post-oxidation sintering, dense RBMO composites have been synthesized with submicrometer grain sizes. For example, Claussen et al.97 have reported the syntheses of reaction bonded composites comprised of 55 vol.% mullite, 10 vol.% silicon carbide, 21 vol.% alumina, and 14 vol.% zirconia with phase sizes well below 1 μm. The introduction of fine (0.2 μm dia.) α-Al2O3 seeds to preform mixtures to enhance the conversion of γ-Al2O3 (a metastable alumina polymorph formed during low-temperature aluminum oxidation) into α-Al2O3, and to further refine the grain size of sintered reaction-bonded alumina, has been suggested by Suvaci and Messing.128 Mullite crystal seeding to reduce the mullite grain size in reaction-bonded mullite has also been proposed by She et al.129 Fine-grained reaction-bonded alumina has been reported to exhibit superplastic-like behavior at 1,250–1,500°C, which may be utilized to generate dense, shaped, nanocrystalline components, via sinter forming or sinter forging, at appreciably lower temperatures than for pressureless sintering.97,101 Bourz et al.130 have generated sinter-forged nanocrystalline (0.63 μm ave. alumina grain size) reaction-bonded alumina-zirconia composites of >95% of theoretical density at only 1,300°C. Such nanocrystalline, reaction-bonded alumina-zirconia composites have exhibited room temperature fracture strengths (in biaxial bending) of 1.1 GPa.130 Dense, fine-grained, reaction-bonded alumina-zirconia composites prepared by pressureless sintering at 1,550°C have also exhibited respectable room temperature fracture strength and fracture toughness values (in 4-point bending) of ≥700 MPa and 3.5 MPa·m1/2.96,100 Dense, fine-grained, reaction-bonded mullite-silicon carbide- alumina-zirconia composites (with 49–55 vol.% mullite) prepared by pressureless sintering at 1,550°C have exhibited room temperature fracture strength and fracture toughness values (in 4-point bending) of 490–610 MPa and 4.1–4.9 MPa·m1/2.97,99 As demonstrated in this overview, nanostructured materials with complex macroscopic-to-microscopic shapes and with tailorable chemistries may be fabricated by first generating a preform with a desired morphology and then chemically transforming the shaped preform into a new material that retains the preform morphology (a type of materials "alchemy"). This fabrication paradigm enables materials that can be readily generated with complex shapes (e.g., formable metal-rich compositions for macroscale components or biologically formed inorganic microscale structures) to be used in the preform fabrication step prior to chemical transformation into the desired material. Under appropriate conditions, gas/solid and liquid/solid reactions can then be used for the shape-preserving conversion of such macroscopic-to-microscopic solid preforms into chemically tailored nanostructures with desired properties. The identification of reaction conditions that allow for such shape-preserving conversion into new nanostructured materials has been significantly aided by utilizing well-known concepts and phenomena from the field of high temperature corrosion (e.g., the Pilling–Bedworth Ratio, active vs. passive corrosion, ignition, internal oxidation). Indeed, by taking the perspective that fluid/solid reactions are a type of "chemical processing", as opposed to a type of "corrosion", this author is confident that scholars who are well-versed in the prior mechanistic knowledge established in the field of high temperature corrosion will continue to develop attractive reaction-based approaches for synthesizing advanced nanostructured materials with tailored morphologies, chemistries, and properties. ACKNOWLEDGEMENTS K.H.S. acknowledges the financial support of the Air Force Office of Scientific Research (Dr. Charles Lee, Dr. Hugh DeLong, and Dr. Joan Fuller, program managers) and the U.S. Department of Energy (Dr. Michael Markowitz, program manager). REFERENCES 1. F.E. Round, R.M. Crawford, and D.G. Mann, The Diatoms. Biology and Morphology of the Genera (New York: Cambridge University Press, 1990).2. D.G. Mann, Phycologia, 38 (6) (1999), pp. 437–495. 3. M. Hildebrand, Chem. Rev., 108 (2008), pp. 4855–4874. 4. V. Martin-Jezequel, M. Hildebrand, and M.A. Brzezinski, J. Phycol., 36 (2000), pp. 821–840. 5. K.H. Sandhage et al., Int. J. Appl. Ceram. Technol., 2 (4) (2005), pp. 317–326. 6. R. Gordon, F.A.S. Sterrenburg, and K.H. Sandhage, J. Nanosci. Nanotechnol., 5 (1) (2005), pp. 1–4. 7. K.H. Sandhage et al., Adv. Mater., 14 (6) (2002), pp. 429–433. 8. K.H. Sandhage, U.S. patent 7,067,104 (27 June 2006). 9. K.H. Sandhage, U.S. patent 7,204,971 (17 April 2007). 10. A.A. Nayeb-Hashemi and J.B. Clark, Bull. Alloy Phase Diagr., 5 (6) (1984), pp. 584–592. 11. M.B. Dickerson et al., J. Nanosci. Nanotech., 5 (1) (2005), pp. 63–67. 12. M.T. Frost et al., Trans. Inst. Mining Metall., 99 (1990), pp. C117–C124. 13. R. Kon and M. Miyazaki, Fragrance J., 31 (8) (2003), pp. 63–68. 14. J.J. Mortvedt and J.J. Kelsoe, Fert. Res., 15 (2) (1988) pp. 155–161. 15. Z. Xin, W.B. Tucker, and R.W. Hemken, J. Dairy Sci., 72 (2) (1989), pp. 462–470. 16. S. Spychaj and F.J. Balta Calleja, J. Mater. Sci. Lett., 12 (16) (1993), pp. 1255–1257. 17. Y. Cai et al., J. Am. Ceram. Soc., 88 (7) (2005), pp. 2005–2010. 18. M.S. Haluska et al., Powder Diff., 20 (4) (2005), pp. 306–310. 19. M.S. Haluska et al., Rev. Sci. Instr., 76 (2005), pp. 126101-1–126101-4. 20. S.M. Allan et al., Ceram. Eng. Sci. Proc., 26 (3) (2005), pp. 289–296. 21. Z. Bao et al., Nature, 446 (3) (2007), pp. 172–175. 22. K.H. Sandhage, U.S. patent 7,393,517 (1 July 2008). 23. K.H. Sandhage and Z. Bao, U.S. patent 7,615,206 (10 November 2009). 24. E.K. Richman et al., Nano Lett., 8 (9) (2008), pp. 3075–3079. 25. R.F. Shepherd et al., Adv. Mater., 20 (24) (2008), pp. 4734–4739. 26. M. Ibisate, D. Golmayo, and C. Lopez, Adv. Mater., 21 (28) (2009), pp. 2899–2902. 27. M. Bengtsson et al., Phys. Status Solidi A, 182 (2000), pp. 495–504. 28. H.-C. Shin et al., J. Power Sources, 139 (2005), pp. 314–320. 29. V. Pacebutas, K. Grigoras, and A. Krotkus, Phys. Scripta, T69 (1997), pp. 255–258. 30. J.R. Szczech and S. Jin, J. Solid State Chem., 181 (7) (2008), pp. 1565–1570. 31. R.R. Unocic et al., Chem. Comm., 7 (2004), pp. 795–796. 32. K.H. Sandhage et al., Handbook of Biomineralization, ed. P. Behrens and E. Bauerlein (Weinheim, Germany: Wiley-VCH Verlag GmbH, 2007), pp. 235–253. 33. S.-J. Lee et al., J. Am. Ceram. Soc., 90 (5) (2007), pp. 1632–1636. 34. S. Shian and K.H. Sandhage, Rev. Sci. Instr., 80 (2009), pp. 115108/1–115108/7. 35. J.C. Lytle et al., Chem. Mater., 16 (20) (2004), pp. 3829–3837. 36. S. Shian et al., J. Am. Ceram. Soc., 89 (2) (2006), pp. 694–698. 37. C. Wagner, J. Appl. Phys., 29 (9) (1958), pp. 1295–1298. 38. S.Y. Ip, M.J. McNallan, and D.S. Park, J. Am. Ceram. Soc., 75 (7) (1992), pp. 1942–1048. 39. T. Hyodo et al., Chem. Sensors, 18 (2002), pp. 178–180. 40. M. Lanata et al., Opt. Mater., 17 (2001), pp. 11–14. 41. C.J. Barbe et al., J. Am. Ceram. Soc., 80 (1997), pp. 3157–3171. 42. M. Manso et al., Biomater., 23 (2002), pp. 349–356. 43. G. Fu, P.S. Vary, and C.-T. Lin, J. Phys. Chem. B, 109 (18) (2005), pp. 8889–8898. 44. J.H. Braun, J. Coatings Technol., 69 (868) (1997), pp. 59–72. 45. A.B. Alles, V.R.W. Amarakoon, and V.L. Burdick, J. Am. Ceram. Soc., 72 (1) (1989), pp. 148–151. 46. M. Viviani et al., J. Euro. Ceram. Soc., 21 (10-11) (2001), pp. 1981–1984. 47. M.A.R.C. Alencar et al., Appl. Phys. Lett., 84 (23) (2004), pp. 4753–4755. 48. D.E. Rase and R. Roy, J. Am. Ceram. Soc., 38 (11) (1955), pp. 389–395. 49. R.S. Roth et al., J. Solid State Chem., 104 (1) (1993), pp. 99–118. 50. M.R. Weatherspoon et al., Chem. Commun., (5) (2005), pp. 651–653. 51. M.R. Weatherspoon et al., J. Electrochem. Soc., 153 (2) (2006), pp. H34–H37. 52. Y. Cai et al., Ceram. Eng. Sci. Proc., 27 (8) (2007), pp. 49–56. 53. R. Zeis et al., J. Power Sources, 165 (1) (2007), pp. 65–72. 54. D. Ding and Z. Chen, Adv. Mater., 19 (15) (2007), pp. 1996–1999. 55. R.W. Ertenberg, B. Andraka, and Y. Takano, Phys. B, 284-288 (2000), pp. 2022–2023. 56. Z. Bao et al., Adv. Mater., 21 (4) (2009), pp. 474–478. 57. E.M. Ernst et al., J. Mater. Res., 22 (5) (2007), pp. 1121–1127. 58. M.C. Breslin, U.S. patent 5,214,011 (25 May 1993). 59. M.C. Breslin et al., Mater. Sci. Eng., A195 (1995), pp. 113–119. 60. G.S. Daehn and M.C. Breslin, JOM, 58 (11) (2006), pp. 87–91. 61. E. del Rio et al., Mater. Sci. Eng. A, 463 (2007), pp. 115–121. 62. R.E. Loehman, K. Ewsuk, and A.P. Tomsia, J. Am. Ceram. Soc., 79 (1) (1996), pp. 27–32. 63. W.G. Fahrenholtz et al., J. Am. Ceram. Soc., 79 (9) (1996), pp. 2497–2499. 64. Y. Gao et al., J. Mater. Sci., 31 (1996), pp. 4025–4032. 65. M.Y. Chen and M.C. Breslin, Wear, 249 (10-11)(2001), pp. 868–876. 66. N. Yoshikawa, A. Hattori, and S. Taniguchi, Mater. Sci. Eng., A342 (2003), pp. 51–57. 67. V.S.R. Murthy et al., J. Am. Ceram. Soc., 88 (10) (2005), pp. 2902–2907. 68. A.C. Strange and M.C. Breslin, U.S. patent 5,728,638 (17 March 1998). 69. J.S. Evarts, M.S. Thesis, The Ohio State University (2008). 70. C. Scheu et al., Phys. Stat. Sol. A, 166 (1998), pp. 241–255. 71. F. Wagner et al., J. Euro. Ceram. Soc., 19 (1999), pp. 2449–2453. 72. P. Beyer, R. Janssen, and N. Claussen, Adv. Eng. Mater., 2 (11) (2000), pp. 734–737. 73. P. Kumar and K.H. Sandhage, J. Mater. Sci., 34 (23)(1999), pp. 5757–5769. 74. K.A. Rogers et al., J. Am. Ceram. Soc., 82 (3) (1999), pp. 757–760. 75. K.H. Sandhage and P. Kumar, U.S. patent 6,407,022 (18 June 2002). 76. M.B. Dickerson, R.L. Snyder, and K.H. Sandhage, J. Am. Ceram. Soc., 85 (3) (2002), pp. 730–732. 77. K.H. Sandhage et al., U.S. patent 6,598,656 (29 July 2003). 78. K.H. Sandhage and P. Kumar, U.S. patent 6,833,337 (21 December 2004). 79. M.B. Dickerson et al., J. Mater. Sci., 39 (19) (2004), pp. 6005–6015. 80. D.W. Lipky et al., J. Euro. Ceram. Soc., accepted, in press. 81. N. Claussen et al., European patent 1,252,349, German patent 10,047,384 (9 August 2001). 82. P. Kumar et al., Scripta Mater., 44 (2001), pp. 751–757. 83. T. Selchert, R. Janssen, and N. Claussen, Ceram. Eng. Sci. Proc., 24 (4) (2003), pp. 175–180. 84. T. Selchert, R. Janssen, and N. Claussen, Ceram. Eng. Sci. Proc., 25 (1-3) (2004), pp. 459–464. 85. R. Janssen, S. Scheppokat, and N. Claussen, J. Euro. Ceram. Soc., 28 (2008), pp. 1369–1379. 86. JCPDS International Center for Diffraction Data File 43-1484 for Al2O3, 21-1276 for TiO2, 45-946 for MgO, 35-0784 for ZrC, 25-1047 for WC, 4-0806 for W (JCPDS International Center for Diffraction Data, Newtown Square, PA, 2007). 87. N. Travitsky et al., Adv. Eng. Mater., 5 (4) (2003), pp. 256–259. 88. N. Travitsky et al., Mater. Sci. Eng., A344 (2003), pp. 245–252. 89. N. Claussen, U.S. patent 5,607,630 (4 March 1997). 90. K.H. Sandhage, U.S. patent 5,318,725 (7 June 1994). 91. K.H. Sandhage, U.S. patent 5,447,291 (5 September 1995). 92. N.B. Pilling and R.E. Bedworth, J. Inst. Met., 29 (1923), pp. 529–591. 93. D. Holz et al., J. Euro. Ceram. Soc., 16 (1996), pp. 255–260. 94. N. Claussen, T. Le, and S. Wu, J. Euro. Ceram. Soc., 5 (1989), pp. 29–35. 95. N. Claussen, N.A. Travitsky, and S. Wu, Ceram. Eng. Sci. Proc., 11 (1990), pp. 806–820. 96. S. Wu, D. Holz, and N. Claussen, J. Am. Ceram. Soc., 76 (4) (1993), pp. 970–980. 97. N. Claussen, S. Wu, and D. Holz, J. Euro. Ceram. Soc., 15 (1994), pp. 97–109. 98. D. Holz et al., J. Am. Ceram. Soc., 77 (10) (1994), pp. 2509–2517. 99. S. Wu and N. Claussen, J. Am. Ceram. Soc., 77 (11) (1994), pp. 2898–2904. 100. R. Janssen and N. Claussen, Ceram. Eng. Sci. Proc., 22 (3) (2001), pp. 51–58. 101. M. Reiterer, T. Kraft, and H. Riedel, J. Euro. Ceram. Soc., 24 (2004), pp. 239–246. 102. S.M. Allameh and K.H. Sandhage, J. Mater. Res., 13 (5) (1998), pp. 1271–1285. 103. K.H. Sandhage et al., Mater. & Manuf. Proc., 15 (1) (2000), pp. 1–28. 104. M.M. Antony and K.H. Sandhage, J. Mater. Res., 8 (11) (1993), pp. 2968–2977. 105. H.J. Schmutzler, M.M. Antony, and K.H. Sandhage, J. Am. Ceram. Soc., 77 (3) (1994), pp. 721–729. 106. H.J. Schmutzler and K.H. Sandhage, Metall. Trans. B, 26B (1995), pp. 135–148. 107. H.J. Schmutzler, K.H. Sandhage, and J.C. Nava, J. Am. Ceram. Soc., 79 (6) (1996), pp. 1575–1584. 108. G.A. Ward and K.H. Sandhage, J. Am. Ceram. Soc., 80 (6) (1997), pp. 1508–1516. 109. S.M. Allameh and K.H. Sandhage, J. Am. Ceram. Soc., 80 (12) (1997), pp. 3109–3126. 110. P. Kumar and K.H. Sandhage, J. Mater. Res., 13 (12) (1998), pp. 3423–3435. 111. S.M. Allameh and K.H. Sandhage, J. Mater. Res., 14 (11) (1999), pp. 4319–4328. 112. D.H. Viers and K.H. Sandhage, J. Am. Ceram. Soc., 82 (1) (1999), pp. 249–252. 113. R. Citak, K.A. Rogers, and K.H. Sandhage, J. Am. Ceram. Soc., 82 (1) (1999), pp. 237–240. 114. E. Saw et al., J. Am. Ceram. Soc., 83 (4) (2000), pp. 998–1000. 115. E. Saw et al., Mater. & Manuf. Proc., 15 (1) (2000), pp. 29–46. 116. M.J. Watson et al., J. Am. Ceram. Soc., 81 (8) (1998), pp. 2053–2060. 117. E. Suvaci and G.L. Messing, J. Mater. Sci., 34 (13) (1999), pp. 3249–3261. 118. K.H. Sandhage and N. Claussen, The Encyclopedia of Materials Science and Technology, Volume 9, ed. K.H.J. Buschow et al. (New York: Elsevier Science Publishers, 2001), pp. 8035–8040. 119. L.J. Masur et al., JOM, 46 (12) (1994), pp. 28–30. 120. K.H. Sandhage et al., J. Am. Ceram. Soc., 79 (7) (1996), pp. 1839–1850. 121. S. Gaus et al., J. Am. Ceram. Soc., 82 (4) (1999), pp. 909–915. 122. M.J. Watson et al., Acta Mater., 49 (6) (2001), pp. 1095–1103. 123. M.J. Watson et al., J. Am. Ceram. Soc., 88 (12) (2005), pp. 3380–3387. 124. X-D. Zhang, K.H. Sandhage, and H.L. Fraser, J. Am. Ceram. Soc., 81 (11) (1998), pp. 2983–2997. 125. X.D. Zhang, K.H. Sandhage, and H.L. Fraser, J. Mater. Res., 13 (11) (1998), pp. 3122–3134. 126. T.J. Detrie and K.H. Sandhage, J. Mater. Res., 15 (2) (2000), pp. 306–316. 127. K.H. Sandhage, J. Electrochem. Soc., 139 (6) (1992), pp. 1661–1671. 128. E. Suvaci and G.L. Messing, J. Am. Ceram. Soc., 84 (3) (2001), pp. 657–659. 129. J. She et al., J. Euro. Ceram. Soc., 22 (2002), pp. 323–328. 130. M.M.R. Boutz et al., Mater. Sci. Eng. A, A233 (1997), pp. 155–166. Kenneth H. Sandhage is the B. Mifflin Hood Professor in the School of Materials Science and Engineering, and Adjunct Professor in the School of Chemistry and Biochemistry, at the Georgia Institute of Technology, 771 Ferst Drive, Atlanta, GA 30332; (404) 894-6882; e-mail: ken.sandhage@mse.gatech.edu. |