LATEST ISSUE |
||||
TMS QUICK LINKS: |
• TECHNICAL QUESTIONS • NEWS ROOM • ABOUT TMS • SITE MAP • CONTACT US |
JOM QUICK LINKS: |
• COVER GALLERY • CLASSIFIED ADS • SUBJECT INDEXES • AUTHORS KIT • ADVERTISE |
|
| Nickel based Superalloys: Research Summary | Vol. 62, No.10 pp. 35-40 |
Metal-modified Nickel based Superalloys
F. Zhang, J. Zhu, W. Cao, C. Zhang, and Y.A. Chang
Questions? Contact jom@tms.org. |
|
Thermodynamic modeling of precious-metal-modified Ni-based superalloys (PMMS) was performed in this study using the CALPHAD approach. With this approach, the effects of platinum-group metals (PGMs) such as platinum, iridium, and ruthenium on the properties of nickel-based superalloys and their interplay with other alloying elements were understood from a thermodynamic and phase equilibrium point of view. Thermodynamic database containing PGMs was developed on the basis of the PanNi1 database for multicomponent nickel alloys. The database was first validated with available experimental data. It was then used to understand phase stability and phase transformation temperatures, such as liquidus, solidus, and γ' precipitation temperature, of PGM modified nickelbased superalloys. The effects of alloying elements on the formation of strengthening γ' precipitate and their partitioning in γ and γ' were also discussed.
As structural materials, nickel-based
superalloys possess outstanding properties
at elevated temperatures and play
an important role for high-temperature
applications, especially in the development
of gas turbine engine components.
The continual demand for improved
performance of jet engines has pushed
the usage of these alloys to even higher
temperatures, which requires better mechanical properties, such as creep
strength. The high-temperature strength
of modern superalloys depends heavily
on the use of refractory alloying elements.
However, the composition of superalloys
must be carefully controlled
since the refractory alloying elements
promote the formation of topological
closed packed (TCP) phases which are
detrimental to the mechanical properties.
On the other hand, while focusing
on the superior mechanical properties,
the environmental stability of these alloys,
such as oxidation and corrosion
resistance, must be addressed due to
the harsh operating environment of gas
turbine engines. It is therefore desirable
to develop nickel-based superalloys,
which are inherently strong, and at the
same time possess good oxidation and
corrosion properties. This is challenging
since optimal alloying compositions for
strength often contradict those needed
for oxidation and corrosion resistance.
This leads to the inevitable use of environmental
barrier coatings (EBCs)
and thermal barrier coatings (TBCs) for
high-temperature applications.
The purpose of this paper is to understand the properties of PGM-modified nickel-based superalloys from a thermodynamic point of view. Phase diagrams, which provide insight into phase stability, are road maps for alloy design and development. Traditionally, phase diagrams have been determined purely by experimentation, which is costly and time consuming. While an experimental approach is feasible for the determination of binary and simple ternary phase diagrams, it is less efficient for the complicated ternaries, and becomes practically impossible for higher order systems over a wide range of composition and temperature. On the other hand, commercial nickel-based superalloys are mostly multi-components in nature, and a more efficient approach is therefore needed to understand the phase equilibria when PGMs are used as alloying elements. The CALPHAD (i.e., CALculation of PHAse Diagram) approach will be adopted in this study. This approach has been used to understand binary, ternary, and even quaternary phase equilibria of nickel-based superalloys including PGMs.4–7 In this study, it will be used to understand the thermodynamics and phase equilibria of PGM-modified multi-component nickel-based superalloys. In this paper, we will first give a brief introduction to the CALPHAD approach and the use of this approach in the development of a multi-component thermodynamic database for nickel alloys containing PGMs. The developed database will then be used to calculate phase transformation temperatures, phase fractions, and other properties for the multi-component nickel alloys containing PGMs and will be validated by the experimental data. Finally, the validated database is used to understand the effects of alloying elements, such as Al, Cr, Pt, Ir, and Ta, and their interplay on the properties of nickelbased superalloys. The CALPHAD approach, which
has been discussed a great deal in the
past several decades,8–11 is a phenomenological
approach. The essence of
this approach is to obtain self-consistent
thermodynamic descriptions of
the lower order systems—binaries and
ternaries—in terms of known thermodynamic
data measured experimentally
and/or calculated theoretically, as well
as the measured phase equilibria. The
advantage of this method is that the
separately measured phase diagrams
and thermodynamic properties can be
represented by the same “thermodynamic
description” of a materials system
in question. More importantly, on
the basis of the known descriptions of
the constituent lower order systems, a
reliable description of a higher order
system can be obtained via an extrapolation
method.12 This description enables
us to calculate phase diagrams of
the multi-component systems that are
experimentally unavailable. Development
of a thermodynamic description
(usually called “thermodynamic database”
or “database) of a multi-component
system therefore starts with the
development of the descriptions for all
the constituent binaries and ternaries.
For example, a quaternary system consists
of six binaries and four ternaries
and the database for the quaternary can
be built up by combining the descriptions
of these six binaries and four ternaries
using a geometric model. It is
found that the binary interactions are
strong, the ternary interactions are less
strong, and the quaternary and higher
order interactions are several orders of
magnitude smaller than those of the binaries.
It is for this reason that a multicomponent
system can be well predicted when the thermodynamic descriptions
for the constituent binaries and
ternaries are well developed. However,
a 20-component system consists of 190
binaries and 1,140 ternaries. Not only
are the assessments of so many binaries
and ternaries not realistic, the lack
of experimental data for some of these
subsystems has made the development
of a complete 20-component thermodynamic
database impossible. As a result,
a reasonable alternative is to focus
on some key systems that are important
for industrial applications and have
abundant experimental information.
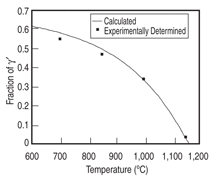
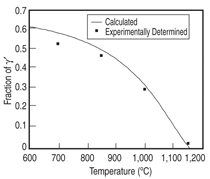
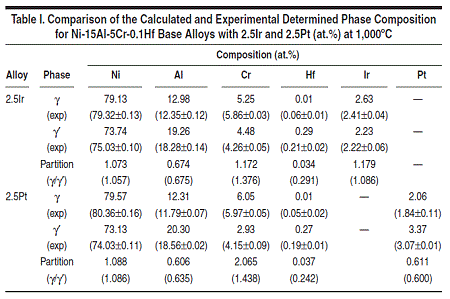
The database was first applied to the Ni-15Al-5Cr-0.1Hf-2.5Pt (at.%) and the Ni-15Al-5Cr-0.1Hf-2.5Ir (at.%) alloys. The fraction of γ' as a function of temperature is calculated for these two alloys and compared with the experimental data13 as shown in Figures 1 and 2. The calculated phase composition of γ and γ' and the partition coefficients for these two alloys at 1,000°C are listed in Table I and compared with the experimental data13 (in parentheses).
As can be seen from these two figures and the table, the calculated phase fractions of γ' agree with the experimental data very well for both alloys, while the calculated phase compositions are also in reasonable accord with the experimental data. The calculated hafnium composition in the γ phase is too low for both alloys, which leads to the strong partitioning of hafnium to the γ' phase. This is because the overall concentration of hafnium is very low and a small variation in either calculation or experimentation will lead to a big difference. For the Ni-15Al-5Cr-0.1Hf-2.5Pt alloy, the measured chromium concentration in the γ' phase is more than 1 at.% higher than that calculated, while the measured chromium concentration in the γ phase agrees with that calculated value very well. The measured chromium concentration in the γ' phase is believed to be too high when mass balance is applied. As can be seen from Figure 1, the calculated and measured phase fraction for the γ' phase match each other perfectly (0.34) at 1,000°C. The overall concentration of chromium is then calculated by the following equation:
By taking the experimental measured
concentration of chromium in the two
phases into the above equation, we get
the overall composition of chromium
to be 5.35 at.% which is higher than its
nominal composition of 5 at.%. On the
other hand, the calculated phase composition
meets the mass balance and
gives the perfect overall composition of
5 at.% Cr.
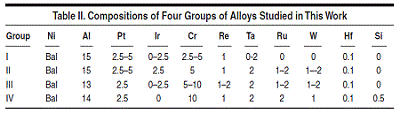
Group I
alloys are based on Ni-15Al-1Re-0.1Hf
(at.%) with Pt varying from 2.5 to 5
at.%, Ir 0 to 2.5 at.%, Cr 2.5 to 5 at.%,
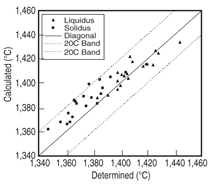
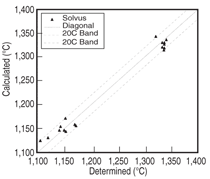
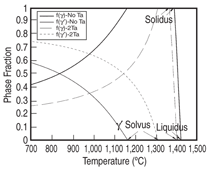
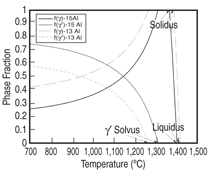
and Ta 0 to 2 at.%. Figure 3 shows the
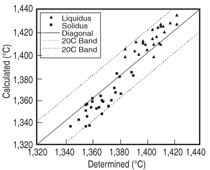
comparison between the calculated and
measured14,15 liquidus and solidus for this
group of alloys, and Figure 4 compares
that of the γ' solvus for the same group
of alloys. It is seen that the calculated
liquidus temperatures agree with the experimental
measured values very well.
The calculated solidus temperatures for
this group of alloys tend to be higher
than those measured experimentally,
while the difference is less than 20°C
for the majority of these alloys. Group
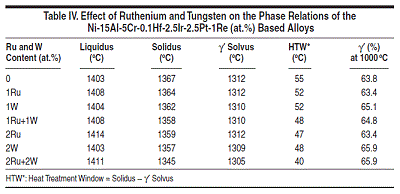
II alloys are based on Ni-15Al-2.5Ir-
5Cr-1Re-2Ta-0.1Hf (at.%) with Pt varying
between 2.5 and 5 at.%, Ru between
1 and 2 at.%, and W between 1 and 2
at.%. The major difference between
Group II and Group I is the use of Ru
and W in Group II. Group III and IV alloys
reduce the Al content while increase
the Cr content as compare to Group II.
Figure 5 compares the calculated and
measured14,15 liquidus and solidus for
Group II-IV alloys. It is interesting to
see that the calculated solidus are lower
than those experimentally determined
values for Group II-IV alloys, which is
contrary to the Group I alloys. Yet, the
agreement between the calculated and
measured phase transformation temperatures
for all these alloys is in general
quite satisfactory. A similar plot for the
γ' solvus was not shown for Group II-IV
alloys due to the lack of experimental
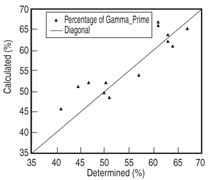
data. Figure 6 compares the calculated
and the measured14 percentage of the
γ' phase for several alloys from Group
II-IV.
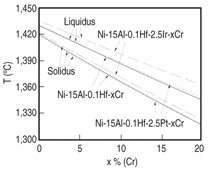
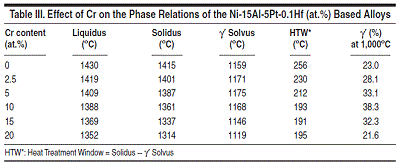
The heat treatment window decreases at
first, and stays at almost constant value
with chromium content greater than 10
at.%. The amount of γ' reaches the maximum
at ~10 at.% Cr, and starts to decrease
as more chromium is added. The
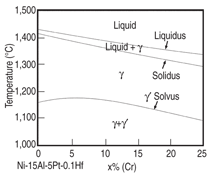
trend can also be illustrated by the phase
diagram shown in Figure 7. As is seen in
this figure, the liquidus and solidus keep
going down as more chromium is added
to the Ni-15Al-5Pt-0.1Hf-based alloy,
while the γ' solvus reaches the maximum
at ~5 at.% Cr. A similar trend is
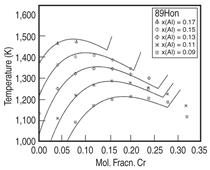
also observed for the Ni-Al-Cr alloys as
shown in Figure 8, which compares the
calculated and experimental measured16
γ' solvus lines for a variety of Ni-Al-Cr
ternary alloys.
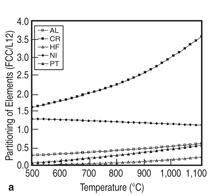
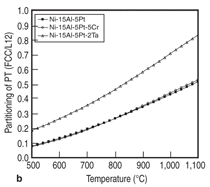
Elemental partitioning, which affects the shape of γ' precipitate and the coherence between γ and γ', plays an important role in determining the mechanical properties of nickel-based superalloys. It is therefore interesting to see how the elemental partitioning is affected by a variety of factors, such as temperature and other alloying elements. Figure 12 demonstrates two examples of such calculations. Figure 12a shows the temperature effect on the elemental partitioning of every element in alloy Ni-15Al-5Cr- 0.1Hf-5Pt. It indicates that temperature has significant effect on the partitioning of chromium for this alloy, while its effect on that of nickel is very small. Figure 12b shows the effects of chromium and tantalum on the partitioning of platinum for the Ni-15Al-5Pt-based alloy. It is seen that 5 at.% Cr has a marginal effect on the partitioning behavior of platinum, while tantalum reduces the partitioning tendency of platinum to the γ' precipitate. The traditional development cycle of nickel-based superalloys using a trial-and-error experimental approach may last for many years due to the difficulty of finding the optimum alloy composition with desired mechanical properties and material stability. Modern material design inevitably involves a computational approach which provides guidance for the selection of alloy chemistry and processing conditions. In this study, thermodynamic calculation is used to understand the phase stability and thermodynamic properties of multi-component nickel alloys containing PGMs, in particular, the calculated liquidus, solidus, and γ' solvus provide the temperature windows for processing. The calculated fraction of γ' precipitate as a function of alloy compositions helps to identify the alloys that have potential for high strength, and the partitioning of elements is used to understand the γ/γ' misfit. These calculations are therefore used to guide the selection of optimum chemistry with balanced properties. For example, alloy chemistry needs to be carefully adjusted so that a high volume fraction of γ' precipitate is obtained for high strength, while a reasonable heat treatment window is maintained. Thermodynamic calculation in combination with key experiments shows great potential for accelerating the process of materials development and optimization. A portion of this work was conducted due to the PMMS project sponsored by the Air Force Research Laboratory (AFRL) in collaboration with Rolls-Royce, University of Michigan, and Iowa State University. The authors acknowledge D. Ballard (AFRL), B. Gleeson (University of Pittsburgh), A. Heidloff (Iowa State University), T. Pollock (University of California at Santa Barbara), J. Van Sluytman (University of Michigan), A. Bolcavage and R. Helmink (Rolls-Royce) for technical discussions. REFERENCES 1. PanNi–Thermodynamic Database for Multicomponent Nickel Alloys (CompuTherm, LLC: Madison, WI, 2000).2. C.W. Corti, D.R. Coupland, and G.L. Selman, Platinum Metals Rev., 24 (1) (1980), pp. 2–11. 3. B. Gleeson et al., Materials Science Forum, 461-464 (2004), pp. 213–222. 4. C. Zhang et al., Acta Materialia, 56 (2008), pp. 2576–2584. 5. J. Zhu, “Thermodynamic Description of Multicomponent Ni-Base Superalloys Containing Al, Cr, Ru, and Pt: A Computational Thermodynamic Approach Coupled with Experiments” (Ph.D. thesis, University of Wisconsin-Madison, 2008). 6. C. Zhang et al., Calphad, 33 (2) (2009), pp. 420– 424. 7. J. Zhu et al., Acta Materialia, 57 (2009), pp. 202– 212. 8. Y.A. Chang et al., Progress in Materials Science, 49 (2004), pp. 313–345. 9. M. Hillert, NATO ASI Ser., Ser. E, 276 (1994), pp. 113–124. 10. L. Kaufman, Computer Calculation of Phase Diagrams (New York: Academic Press, 1970). 11. N. Saunders and A.P. Miodownik, CALPHAD: A Comprehensive Guide, ed. R.W. Cahn (New York: Pergamon Materials Series, 1998). 12. K.-C. Chou and Y.A. Chang, Ber. Bunsenges, Phys. Chem., 93 (1989), p. 735. 13. A.J. Heidloff et al., Metallurgical and Materials Trans. A, 40 (2009), pp. 1529–1540. 14. J.S. Van Sluytman and T.M. Pollock, Private Communication (University of Michigan at Ann-Arbor, 2009). 15. J.S. Van Sluytman et al., Acta Materialia, 58 (2010), pp. 1952–1962. 16. Y.M. Hong et al., ISIJ Int., 29 (1) (1989), pp. 78–84. F. Zhang and W. Cao are with CompuTherm, LLC, Madison, WI; J. Zhu is with the Department of Materials Science and Engineering, University of Michigan, Ann Arbor, MI; and C. Zhang and Y.A. Chang are with the Department of Materials Science and Engineering, University of Wisconsin, Madison, WI. Dr. F. Zhang can be reached at fan.zhang@computherm.com. |