|
To overcome limitations in current
technology and to open the door to
breakthroughs in the extraction and recycling
of metals, melt circulation
within closed loops is proposed. The
general features of generic melt circulation
technology, particularly for massive
reductions in energy consumption,
are highlighted. Reference is made to
the recently published paper on lower-energy
primary aluminum. More detailed
attention is then focused on coproduction
of steel and titanium metal
directly from ilmenite concentrates.
The energy consumption is projected to
be less than one third of the best available
technology (Kroll process). Next,
new copper smelting concepts based on
melt circulation are introduced because
current advanced processes are judged
to be, without exception, energy inefficient.
INTRODUCTION
The metal-producing industries are
responsible for significant greenhouse
gas emissions globally. Also, in cost
terms, there is increasing awareness
that efficient use of energy in general
must be a top priority. Since retiring
from the University of Birmingham
(United Kingdom) in 1998, the author
has made a concerted effort to address
these issues by conference presentations
and published technical papers.
The focus has been on the metal industries,
not only in extraction of metals
from primary resources, but also in recycling
secondary materials, such as
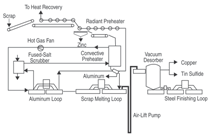
steel scrap, as illustrated in Figure 1.1
BACKGROUND
An overview of generic melt circulation
technology was presented at a Canadian
Institute of Mining, Metallurgy
and Petroleum conference in 19962 and
prior to this a short review article was
published on the same subject in 1994.3
More recently in 2003, a general paper
on melt circulation and conductive
heating was published.4 However, the
origins of the technology can be traced
back much further to the published account
of discussion following a presentation
on coal-based ironmaking.
|
HOW WOULD YOU...
|
…describe the overall significance
of this paper?
Considerable savings in energy
stem from forced circulation of
melts within closed loops to transfer
sensible heat from exothermic
regions to zones requiring thermal
energy input. Prime examples
include carbothermic production of
aluminum and simultaneous direct
continuous recovery of titanium
and iron as metals from ilmenite
concentrates.
…describe this work to a materials
science and engineering professional
with no experience in your
technical specialty?
Provided sub-surface nucleation
and growth of gas bubbles are
precluded by appropriate design
and due account is taken of high
thermal conductivity and corrosive
attack on melt containment,
pyrometallurgical recovery of
metals can be considered as
chemical engineering under
extreme conditions. From this
viewpoint, forced melt circulation
opens the door to exciting
possibilities, which are just not
available with established
high-temperature processing.
…describe this work to a layperson?
For recovery of metals from mineral
and secondary resources, one option
is to employ high temperatures,
where chemical reactions are
inherently rapid. In this context,
forced melt circulation in a closed
loop allows heat to be added in one
zone of a processing reactor and
then to be transported to where
the thermal energy is needed.
This results in effective energy
utilization and allows the
introduction of processing options
not possible with existing
technology.
|
Coal-based ironmaking using the
smelting reduction approach was pioneered
in Sweden. The “father” of
smelting reduction is widely acknowledged
to be Sven Eketorp, a professor
with the Royal Institute of Technology,
Stockholm. Eketorp presented a paper
on the “direct use of coal for production
of molten iron” to a conference in
London in 1981.5 He cited four different
processes attempted in Sweden.
They all failed. Eketorp’s published response
to a question6 that the author
asked in 1981 was: “In reply to Professor
N A Warner, there is very little hope
of finding a process whereby we can
deliver energy to the bath directly, i.e.
by burning CO to CO2 . . .”. He went on
to say: “If energy is produced by an
oxidizing reaction such as combustion
of CO, the problem is to separate heat
transfer and mass transfer. The lining
could not withstand heat pulsation and
the FeO rich slag. . .”.
Obviously the present author does
not accept either of the above statements
as the final word on smelting reduction.
However, in attempting to assess
the likelihood of new ironmaking processes replacing the iron blast furnace
it is necessary to keep Eketorp’s
comments in mind and to realize that to
date there has been a whole catalogue
of unsuccessful attempts. Without exception
the reactors employed have all
been incapable of complete so-called
“post combustion” of CO to CO2 within
the ironmaking reactor itself without
overheating and damaging the refractory
lining and thus making the process
inoperable. The accumulation of slag
within the reactor is the root cause of
the problem. Slag accumulation creates
a thermal barrier, which inhibits efficient heat transfer back to the site of the
endothermic iron producing reaction in
the liquid metal bath.
Melt circulation at a rate many times
the production rate of metals allows
operation of two side-by-side furnace
hearths at slightly different levels so
that melt overflows continuously from
one to the other. If the higher hearth is
where, for example, oxygen top blowing
takes place, this provides the mechanism
for floating slag away as soon as
it is formed without allowing an appreciable
layer thickness to ever build up.
Melt circulation also provides the
means for maintaining one side of the
reactor under neutral or reducing conditions
while oxygen is blowing on the
other. Furthermore, melt circulation allows
the transference of heat from exothermic
reactions (such as iron slagging
and copper conversion) to the endothermic
site (such as zinc gas formation
and charge assimilation) by using sensible
heat transported by the circulating
melt. In addition, fuel can be combusted
and energy can be transmitted directly
at high intensity to what is effectively
a slag-free surface. Also forcibly
circulating a melt within a reactor overcomes
limitations inherent in conventional
pyrometallurgical furnaces such
as back-mixing and non-countercurrent
contacting.
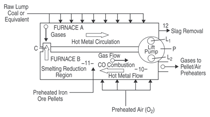
A proposed melt circulation approach
for continuous coal-based ironmaking
is shown in Figure 2.7 The remaining
challenge facing the ferrous
industry is the introduction of an energy-efficient integrated process for continuous
steelmaking using coal and
iron ore fines as feed material and ending
up with ultra-low carbon steel, if so
desired. In 2003, the author published three papers on this subject using generic
melt circulation technology.8–10
The key requirement is to balance gas
phase mass transfer, interfacial chemical
kinetics, and liquid phase mass
transfer in order to prevent homogeneous
nucleation of CO beneath the
melt surface. H. Bessemer himself, in
his autobiography paints the picture of
what happens if this is disregarded. Regrettably,
failure to ameliorate CO bubble
eruptions has contributed to a lack
of success in all previous attempts with
continuous steelmaking.
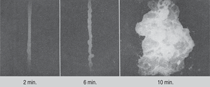
To emphasize just how disastrous
sub-surface nucleation and growth of
CO bubbles can be, Figure 3 shows the
explosive disintegration of molten steel
under stream vacuum degassing conditions,
following CO/CO2 (10/1) gas injection
at the same rate for the time intervals
indicated. Clearly, such high
reaction intensity has to be moderated
if successful continuous steelmaking is
ever to be achieved, even if this means
larger surface area reactors have to be
employed. On the other hand, natural-gas-based steelmaking using iron-ore
fines eliminates the need for any decarburization
whatsoever.11 Refined molten
iron is produced directly with carbon
transfer to the melt prevented by a
thin layer of molten iron oxide. Conventional
oxygen steelmaking as such
is eliminated and sub-surface nucleation
and growth of CO is thus longer
an issue.
MATTE CIRCULATION TRIALS
High-temperature semi-pilot-scale
trials were conducted at the University
of Birmingham employing a closed
loop molten matte circulation system at
temperatures around 1,200°C–1,250°C.
Use was made of a gas-lift system
closely resembling RH steel vacuum
degassing technology. Ruhrstahl Heraeus
(RH) steel degassing is a mature
technology used worldwide for batch
vacuum degassing of liquid steel. Melt
merely overflowed from a high-level
hearth into a lower-level hearth to be
pumped back with the RH-type device.
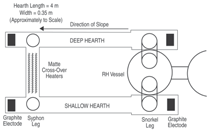
Figure 4 is a schematic plan view of the
plant, which consisted of two refractory-lined rectangular hearths side by
side but at different levels, with a vacuum
lift pump (RH system) connecting
the hearths at one end to transfer matte
from the lower to the upper hearth. A
passage at the other end was to allow
matte to flow back to the lower hearth
under gravity, thus completing the
closed loop. The hearths were contained
within a stainless steel furnace
shell with a detachable lid with a sand
seal around its perimeter to make it effectively
gas tight. The furnace was
vented to a caustic soda scrubber for
removal of sulfur dioxide, water vapor,
and other gaseous products. The furnace
shell was force cooled with ducted
air to ensure that the melt freeze line was within the inner magchrome brick,
which was backed by magnesite and
super-duty firebrick. The scale of the
operation can be gauged from the plant
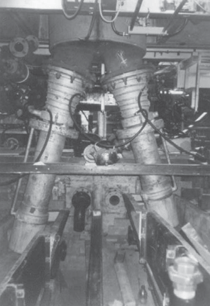
photographs shown in Figures 5 and 6.
The twin hearths contained a known
amount of copper-saturated copper sulfide matte, or “white metal.” The temperature
of the matte was raised to
about 1,250°C by means of direct-resistive
or so-called conductive heating.
Electric currents as high as 7,000 A
were passed through the copper matte
between graphite electrodes at either
end of the hearths. Power to each hearth
was supplied by two 180 kVA welding
transformers with parallel primary
windings, and with their secondary
windings in series, in order to supply
sufficient voltage, and thus power, to
melt the matte. The hearths were designed
so the matte depth for the lower
hearth, at about 280 mm, was greater
than the upper hearth at 150 mm.
The RH-type vacuum system connected
the hearths via two inclined
snorkel legs, which were lowered into
the molten matte immediately prior to
circulation. Inert gas (nitrogen) was injected
into the up leg of the vessel (in
the deep, lower hearth), which as it expanded
forced the liquid upward under
a two-phase, bubble flow regime, into
the main RH chamber. Under vacuum,
the entrained gas was evolved and the
melt returned to the shallow, higher
hearth through the other leg by gravity.
The vacuum system was provided
by a mechanical rotary pump/booster
combination. Various water and nitrogen
cooling circuits served to prevent
overheating of critical components.
Incorporated into the plant was a vacuum-
activated system for emptying
the molten matte from the hearths into
a holding vessel, or dump tank, via a
siphon pipe system. Initially it was proposed
that this dumping system would
be employed after every trial and the
hearths refilled with matte. This did not
prove necessary, as it was shown possible
to re-heat solidified matte in-situ
without adverse effects.
Over a six-hour period some 300
tonnes to 500 tonnes of matte were
circulated past a given point inside a
closed loop comprised of two interconnected
side-by-side open channels,
each 0.35 m wide × 4.0 m long. This
constitutes proof of concept not only of
melt circulation but also electrical conductive
heating for melting the crushed
solid matte charged initially into the
channels and the ability of this mode
of heating in keeping the melt in a molten
state over the six-hour period of the
trial. Accounts of this trial and associated
developments have been published
elsewhere.4,12
NEW HORIZONS
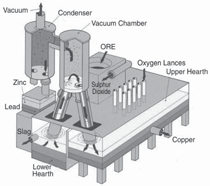
In the non-ferrous field, the original
melt circulation research was targeted
at direct smelting of complex sulfides to
produce zinc, lead, and copper simultaneously
in the one reactor.13 Gradually
the emphasis changed to direct smelting
of bulk zinc-lead concentrates and
finally to zinc concentrates in general.12
The technology has received some notoriety
for proposed zinc metal production,
due principally to the efforts of
P.M.J. Gray,14–16 who aptly referred to
the zinc adaptation as the “Warner Process,”
but the process remains commercially
unproven. The general features
are illustrated in Figure 7.
The major waste product or solid
emission from smelting is slag. Given
appropriate technology, it can be argued
that the natural products to be derived
from copper minerals are metallic
copper and metallic iron. Reduction
of iron in slag is a highly endothermic
chemical reaction, so clearly the possibility
exists of using the excess heat
generated in direct oxygen smelting to
sustain the iron production reaction.
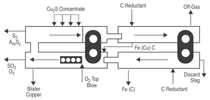
A melt circulation reactor employing
twin loop circuits is proposed, one for
copper matte and the other for fayalite
slag, which in principle could eventually
lead to low energy simultaneous
production of copper and iron with
virtually zero solid-waste generation.17
The general features are shown in Figure
8.
The examples quoted so far have
been well documented and will not be
considered further in the present paper.
Hopefully, there are now improved
prospects for ultimate commercialization
of energy-saving technologies. Together
with perceived threats of global
warming and climate change, the stage
is set for a major multi-national company
to get involved in the generic
technology. The philosophy now is to
intentionally seek out what can only
be referred to as true breakthroughs
with radically innovative technology to
tackle some of the more obvious shortcomings
of the presently accepted status
quo.
There is most certainly a need for an
improved aluminum process. Titanium
metal will not fulfill the ambitious expectations
projected for it over many
years, unless there is a real breakthrough.
The proposed technology for
continuous smelting of ilmenite directly
to titanium metal potentially offers
energy consumption less than one third
of the current best available technology
(Kroll process). This is before claiming
a credit for the co-produced liquid
steel.
Primary zinc metal production is
currently tied too closely to electrowinning. Thus cheap electrical power is essential.
This is unlikely to be available
at the mine site without massive capital
investment, requiring amortization over
periods probably greater than the life of
the mine itself.
Finally, and probably unexpectedly,
there is great scope for improving the
energy efficiency of copper smelting.
The current cutting-edge copper smelting
processes have made tremendous
gains over the traditional processing
route employing reverberatory furnaces
and Peirce-Smith converters. They are,
however, without exception, extremely
energy inefficient.
Generic melt circulation offers the
prospects of major breakthroughs in
aluminum, titanium, zinc, and copper
production. The conceptual design
aspects of two of these advances will
now be considered. The melt circulation
process for aluminum has been detailed
already this year.18 Also, exciting
prospects for massive reduction in the
energy requirements for primary zinc
have been identified based on generic
melt circulation technology. It would
be inappropriate to summarize these in
the present paper, as a manuscript on
the theoretical foundations has been
submitted to Metallurgical and Materials
Transactions.
CO-PRODUCTION OF STEEL AND TITANIUM
Continuous smelting of ilmenite is
proposed, eliminating chlorine-based
technology except perhaps as a means
for dealing with associated radioactivity.19 Concentrates are fed into the
first of three melt circulation loops, in
which optionally both the oxidic melt
and the liquid steel layers may be circulated.
The molten iron formed joins
the circulating bottom layer of liquid
steel, which extracts impurities including
chromium, silicon, manganese,
vanadium, niobium, aluminum, and
phosphorus. Liquid steel is withdrawn
for refining prior to continuous casting.
The oxidic melt has its carbide content
increased in the second of the melt
circulation loops. Continuous vacuum
refining is then conducted in the third
loop operating at 10–4 MPa using a vacuum
steel degassing steam jet ejector
systems, yielding a theoretical (TiO2 +
ZrO2) equivalent in the titanium oxycarbide
melt of “four nines” (99.99%)
purity at 84% recovery.
F. Cardarelli20 describes a method
for electrowinning titanium metal or
alloy from titanium oxide containing
compounds in the liquid state, which is
claimed to have significant advantages
over other emerging technologies. It
involves direct electrowinning of titanium
from molten titanium mixed oxide
compounds. The preferred electrolyte
is molten calcium fluoride. During
the electrochemical reduction, droplets
of liquid titanium metal are produced
at the oxide/electrolyte interface and
sink by gravity, settling to the bottom
of the electrochemical reactor, forming
after coalescence a pool of liquid titanium
metal or alloy. The liquid metal is
continuously siphoned or tapped under
an inert atmosphere and cast into dense
titanium metal ingots.
The initial production of metallic
droplets of impurities such as metallic
iron and other transition metals more
noble than titanium (e.g., manganese,
chromium, vanadium, etc.) detracts seriously
from the process in that cross-contamination
would inevitably occur
between the initial impure metal and
the product titanium. Also, the process
is not truly continuous, a shortfall self-evident
in the batch charging of molten
titanium slag or other molten materials
into the electrochemical reactor.
A truly continuous process is not
yet available which is capable of accepting
ilmenite mineral concentrates
or titaniferous magnetite at one end of
the spectrum right through to synthetic
rutile or solid upgraded titanium slag at
the other. Their transformation in-line
to high-purity liquid titanium II oxide
as the preferred continuous feed for titanium
metal production is the essence
of the proposed melt circulation route to titanium metal. At the same time, it
is desirable to co-produce liquid steel
in a state ready for continuous refi ning
in advance of continuous casting.19
The principle enunciated for smelting
right up to the engineering limit of
structural graphite, say 2,200°C in terms
of stability and mechanical strength, is
that ultra-high-temperature operations
must be conducted relatively close to
thermodynamic equilibrium between
the phases in contact with each other.
Normally in pyrometallurgy these
phases are molten slag, molten metal,
the solid in contact therewith, and the
associated gas phase. Of these, the interaction
of the slag and solid phase is
crucial but the liquid metal/solid hearth
contact must also be considered.
It is accepted that relatively large areas
are required if close to equilibrium
conditions are to apply, and this implies
the use of what have been termed previously
by the author as “swimming pool
reactors.” It is, of course, quite obvious
that swimming pool reactors can only
be entertained if the processing technology
is truly fully continuous without
cyclic variation and with provision
for the withdrawal upward of certain
plant items when processing is interrupted
before the melt freezes over.
For large swimming-pool-sized reactors,
monolithic linings composed
of various grades of titanium oxycarbide
are needed. The ductile-to-brittle
fracture transition for such materials is
very favorable and they would appear
to be able to operate over a temperature
range from above 2,000°C to, say,
800°C. This facilitates maintenance of
the unmelted shell rather than attempting
to use so-called skull formation
with water-cooled hearths. Transition
metal carbides have the ability to deform
plastically above a given temperature,
referred to as the ductile-to-brittle
transition temperature. Below that
temperature titanium carbide fails in a
brittle manner, while above it, it shows
ductile behavior and undergoes plastic
deformation. For TiC this is in the region
of 800°C and because of the cubic
structure of titanium oxycarbide over
the whole range of solid-state stability,
high temperature linings of titanium
oxycarbide can reasonably be expected
to behave in a similar fashion.
This is extremely helpful in terms
of accommodation of thermal stresses
resulting from thermal expansion without
fracturing or forming cracks in the
lining, which could lead to problems
with melt containment and is also very
desirable in maintaining the electrical
and thermal conductivity integrity of
the titanium oxycarbide solid lining.
Water-cooling with traditional skull
formation, on the other hand, does not
secure these beneficial attributes as the
region of brittle fracture replaces plastic
deformation once the temperature
drops much below the critical transition
temperature.
For ilmenite processing, the first of
the sub-processes is the formation and
recovery of a molten iron alloy for subsequent
continuous processing to liquid
steel ready for continuous casting.
Thus a gas phase and two liquid phases
are involved. All of these must be essentially
at equilibrium with each other
throughout the associated melt circulation
loop. The reactor hearth, walls,
and contact areas of equipment such
as lances, snorkels, and overflow and
underflow weirs immersed in the melt
must all be prefabricated from material
of the solidus composition.
Liquid phases must be close to the
liquidus temperature and if two liquid
phases are involved, it follows that
composition and temperature gradients
within the bulk phases must also
be eliminated and preferably each independently
circulated under turbulent
flow conditions to promote good mixing.
This leads to what is believed to be
a totally new approach to pyrometallurgy:
forced circulation of both slag and
metal phases at relatively high rates.
Conducting pyrometallurgical operations
in relatively low-intensity reactors
rather than using high-intensity
reactors currently in vogue presents opportunities
just not available in compact
reactors. Radiative post combustion is
an important case in point. Admittedly,
reactors of Olympic swimming pool
dimensions are going to be needed for
very large-scale operations. If these
large reactors are lined with unmelted
solid shells of the material being processed, the cost implications can be assessed
in terms of the interest lost on
the cash flow not realized because of
the hold-up of product within the process.
This has to be balanced against
the costs involved in conventional refractory
lining of the reactors and the
fact that unmelted shells of product
material are indestructible. They can
be replenished in situ during continued
operations by controlled melting or
freezing, employing electro-conductive
heating in conjunction with steam
rising, or other heat removal means at
high temperature.
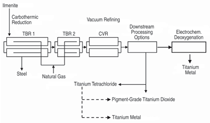
Figure 9 is an overview of the plant
for continuous smelting of ilmenite
concentrates employing three melt circulation
loops in series to feed an electrochemical
deoxygenating reactor to
produce titanium metal.
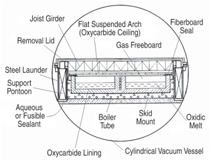
Figure 10 shows a sectional elevation
of a melt circulation loop containing a
single oxidic melt liquid phase showing
the “cavity-wall” type of construction.
This construction is comprised of
an inner hot face lining of solidus composition
titanium oxycarbide, a free
space containing support skids to allow
unimpeded thermal expansion and contraction
of the lining, boiler tubes for
steam raising, superheating or closedloop
steam reheating service as appropriate
on safety grounds, so positioned
that they receive direct thermal radiation
from the cooler face of the oxycarbide
lining, and an outer backing of
conventional refractory and insulating materials, all encased externally in a
gas-tight steel shell.
Special measures need to be taken in
the design of such furnaces to accommodate
differential expansion internally
while keeping the outside surfaces
of the furnaces moderately cool. Freedom
of the hearth to expand or contract
without excessive friction is crucial to
the success of the proposed continuous
smelting technology. Conventional
skid mounting on heat-resistant alloy
shells encasing the cooler faces of the
oxycarbide linings for mechanical integrity
and structural stability, or perhaps
more sophisticated “bogey” rail
tracking may be necessary for this
purpose so that the rather long hearths
involved can expand or contract freely.
In this connection it must be borne in
mind that unscheduled shutdowns have
to be accommodated and the prospect
of the hearths cooling to room temperature
must be addressed at the design
stage. Also, sufficient clearances must
be provided inside the furnace interiors
to permit free expansion and contraction
to take place differentially with
respect to the outer steel encasement or
associated pressure/vacuum vessels.
The refractory roof and its associated
structural steel work is supported on
steel pontoons floating in launders or
troughs on each side of the hearth containing
the melt and extending the full length of the furnace. By pumping liquid
in and out of these launders, the
pontoons can be made to float and thus
during heating up from room temperature
to, say, 2,000°C at the hot face of
the lining, the structure is free to expand
both longitudinally and laterally
across the width of the hearth. When
operating temperature is reached, liquid
can be partially removed from the
troughs so that the pontoon-supported
structures no longer float but rather can
weigh down onto refractory fiberboard
in a controlled fashion to form a gastight
seal. If the plant is to be shut down
from operating mode, the pontoons can
be floated again by pumping liquid
back into the troughs so that the roof
structure and its associated refractory
flat arch can return eventually to the
cold position.
ENERGY-EFFICIENT COPPER SMELTING
Highly intensive reactors, such as
Noranda, Tienente, Mitsubishi, Isasmelt,
and Ausmelt do not capture effectively
the inherent energy in mineral
sulfide concentrates, which is clearly
vital for energy-efficient processing.
Vast amounts of energy are wasted.
Current technology, therefore, normally
needs a degree of oxygen enrichment
to ensure autogenous operations even
when smelting high-energy-content
copper or nickel concentrates. Ideally,
when such materials are smelted in isolation,
excess energy should be available
for recovery by steam-based electric
power generation to satisfy in-plant
requirements and possible export to national
grids, if such are available.
According to U.S. patent 5,607,495,21
heat generated during melt circulation
smelting can be efficiently utilized by
smelting copper/nickel sulfide ore concentrates
of high intrinsic energy value
with another mineral concentrate of
low or negative intrinsic energy value
(e.g., high-grade zinc concentrate,
high-grade lead concentrate, or even a
bulk flotation concentrate containing
both lead and zinc, preferably low in
gangue oxides for highest thermal efficiency). With such a process, metallic
copper, metallic zinc, and metallic lead
can all be obtained as products in the
primary smelting circuit employing
forced circulation of copper/nickel sulfide through various extraction zones.
The zinc and/or lead formation reactions
consume thermal energy and so if
the ore concentrates are added in the
correct proportions, the excess energy
released on direct smelting of copper/
nickel concentrate using technically
pure oxygen can be balanced against
the endothermic requirements of zinc
and/or lead production. This has the
advantage that the energy required for
zinc and lead production is provided in
situ within the smelter so that no external
fuel is required and all the benefits
of virtually zero gas emission smelting
are secured. Preferably, copper is extracted
as the metal, while nickel is extracted
as high-grade nickel sulfide.
In contradistinction to the preceding
paragraphs, it is now considered that
for single copper sulfide concentrate of
high intrinsic energy value, use of oxygen
enrichment or technically pure oxygen
should be avoided in the interest
of energy efficiency if the sole purpose
is to produce copper in the most efficient way possible.
Notwithstanding energy considerations,
it is recognized from the outset
that a move away from high-intensity
reactors could significantly increase
the amount of saleable material held up
within the process. For example, if
there is a significant increase in the
hold-up of molten copper matte within
the circuit, this will have immediate financial implications. Interest will be
lost on the equivalent value of copper
metal not sold to customers. Clearly, to
minimize the hold-up of matte, the first
prerequisite, if large surface-area reactors
are dictated on other grounds, the
flowing melt streams must be very shallow.
In the present context, for smelting
high-intrinsic-energy sulfide, emphasis
is directed toward melt depths in topblown
zones not to exceed about 5 cm
to 10 cm in swimming pool reactors.
The corollary to the above is that top
air blowing must avoid jet penetration
into the melt and be conducted in what
is known as the non-splash mode. The
critical conditions necessary to enforce
this requirement have been the study of
numerous research projects over the
years. For the present purpose based on
available data for melt properties and
likely scenarios for multiple top jetting
of molten cuprous sulfide, the critical
depth of the melt cavity beneath each
jet before splashing commences is in
the region of 1.5 cm to about 1.75 cm.
Recently M. Campforts et al.22 have
expressed the view that the formation
of a freeze layer has to be guaranteed
for high-intensity processes conducted
in furnaces with refractory walls. Such
an approach is compatible with expert
opinion, as expressed by K.M. Donaldson
et al.23 for modern high-productivity
pyrometallurgical furnaces, “. . . bottom leaks invariably lead to catastrophic
run-outs and because the
hearth brickwork is not readily accessible
for repairs, proper attention to the
design and erection of the hearth arches
is the single most important aspect of
furnace construction.” In the context of
continuous copper smelting, this means
that conditions need to be established
by appropriate design to ensure a freeze
lining of cuprous sulfide is maintained throughout to protect the hearth or other
melt containment walls in their entirety.
Also, as matte oxidation generates
iron-oxide-containing slags, the
sidewalls clearly need protection by a
freeze lining, which is preferably contiguous
with that on the hearth or containment
vessel.
For low-intensity continuous copper
smelting, there are two principal melt
circulation loops. The first essentially
replaces the continuous high-intensity
matte-producing reactor systems of
current technology. To ensure effective
removal of minor impurity elements
such as arsenic, antimony, and bismuth,
this first melt circulation loop has a carrier
melt of cuprous sulfide with only a
relatively minor level of ferrous sulfide
but most importantly a relatively small
thermodynamic activity of dissolved
copper metal. This is where the incoming
moderately preheated (before chalcopyrite
decomposition) copper concentrate
feed is dispersed into the slag,
produced initially on the oxidizing side
of the melt circulation loop, and then
floated into the neutral or reducing side.
To effect dispersion, mechanical agitation
or alternatively inert gas sparging
is required and then phase disengagement
must be achieved by gravity separation,
possibly enhanced by electromagnetic
means. This is an extremely
effective procedure for reducing the
copper oxide dissolved in the slag and
can be regarded as in-situ slag cleaning.
The melt is then returned to the matte
oxidation zone containing the extensive
array of air top-blowing jets. It is crucially
important that the positioning of
the jets in terms of spacing and height
above the shallow melt surface be such
that in the region of 80–90% oxygen
utilization is achieved whilst operating
in the non-splash mode.
The second melt circulation loop replaces
the multiple-batch Peirce–Smith
converters of the traditional converter
aisle and it again is low intensity in
terms of the air top-blow arrangements.
The matte in this loop is copper-saturated
and a separate bottom layer of
molten copper is formed. This flows by
gravity down a gently sloping hearth of
frozen cuprous sulfide so that it does
not accumulate in the shallow hearth
itself but rather is collected as a pool
down one end. In the event of a temporary
shutdown, electrical conductive
heating can keep the extensive shallow
layer of molten cuprous sulfide matte,
again about 5 cm or so in depth, in the
liquid state until melt circulation is resumed.
Molten copper siphoned out or otherwise
withdrawn continuously from
the accumulated pool becomes the feed
to two relatively intense contactors in
series based on non-wetted irrigation
of packed beds. Counter-current flow
of inert gas with the controlled minor
addition of air in the first is followed
then by reformed natural gas as the
continuous phase in the second packed
bed.
The purpose of the first contactor is
to remove the residual sulfur in the
blister copper by the reaction S + 2O =
SO2(g), whilst the second reduces residual
oxygen down to specification limits
by the two reactions: O + H2(g) = H2O(g)
and O + CO(g) = CO2(g), where the underlining
represents the elemental species
dissolved in liquid copper.
The gas phase is maintained as a
continuum and high-intensity heat and
mass transfer are achieved by allowing
a liquid-metal stream to disintegrate
and flow downward as droplets or rivulets
by gravity, countercurrent to an upward
flow of gas within a bed of solid
packing material.
The design of the reactor for melt
deoxidation must take the possibility of
longitudinal mixing in the gas phase
fully into account, because countercurrent
conditions are vital in the interests of low natural gas consumption and
hence retention of high energy efficiency.
In this reactor the two liquid phases
involved are molten cuprous sulfide
and copper metal under strongly reducing
conditions. It is well established
that neither of these melts are aggressive
toward high-alumina refractory or
spinel direct-bonded brick (71% Al2O3;
28% MgO) SP. Also C.A. Gonzales et
al.24 have reported that there is zero
penetration of Cu2S into SP at 1,300°C
and pO2 = 10–8 MPa. About Al2O3, R.
Parra et al.25 state that the contact angle
of molten Cu2S on Al2O3 at 1,200°C is
105° and hence this system exhibits
non-wetting behavior. For melt deoxidation
in continuous copper smelting,
it is difficult to imagine a better system
than straightforward gas/liquid contacting
employing a compact and well-insulated
packed bed with true countercurrent
driving forces throughout.
ACKNOWLEDGEMENTS
The research behind the developments
described in this paper has been
funded by the Science and Engineering
Research Council, the Department of
Trade and Industry, the Commission of
the European Communities, the British
Technology Group, the Mineral
Industry Research Organisation, and
the Engineering & Physical Sciences
Research Council.
REFERENCES
1. N.A. Warner, “Continuous Oxygen Steelmaking
with Copper-, Tin-, and Zinc-Contaminated Scrap,”
Metall. and Materials Transactions B, 35 (4) (2004),
pp. 663–674.
2. N.A. Warner, “Overview of Generic Melt Circulation
Technology,” Challenges in Process Intensification,
ed. C.A. Pickles, P.J. Hancock, and J.R. Wynnyckyj
(Montreal, Canada: The Metallurgical Soc. of the
Canadian Institute of Mining, Metall. and Petroleum,
1996), pp. 273–281.
3. N.A. Warner, “Generic Melt Circulation Technology,” Trans. Instn. Min. Metall. (Section C: Mineral Process
Extr. Metall.) (1994), p. 103.
4. N.A. Warner, “Conductive Heating and Melt
Circulation in Pyrometallurgy,” Trans. Inst. Min. Metall.
C, 112 (December 2003), pp. C141–C153.
5. S. Eketorp, O. Wijk, and S. Fukagawa, “Direct Use
of Coal for Production of Molten Iron,” Extraction
Metallurgy ’81 (London: Inst. Min. Metall., 1981), pp.
184–192.
6. S. Eketorp, “Report of Discussion,” Extraction
Metallurgy ’81 (London: Inst. Min. Metall., 1981), p.
D42.
7. N.A. Warner, “Coal-based Ironmaking via Melt
Circulation,” Metallurgical Processes for the Year 2000
and Beyond (Warrendale PA: TMS, 1989), pp. 669–
719.
8. N.A. Warner, “New Reactor Concepts for Direct
Coal-based Continuous Steelmaking,” Metallurgical
and Materials Processing Principles and
Technologies—Proc. Yazawa Int. Symp., ed. F. Kongoli
et al. (Warrendale, PA: TMS, 2003), Vol. 1, pp. 881–
900.
9. N.A. Warner, “Towards Coal Based Continuous
Steelmaking Part 1—Iron Ore Fines and Scrap to Low
Carbon Steel via Melt Circulation,” Ironmaking and
Steelmaking, 30 (6) (December 2003), pp. 429–434.
10. N.A. Warner, “Towards Coal Based Continuous
Steelmaking Part 2—Low Carbon to Ultra Low Carbon
Steel,” Ironmaking and Steelmaking, 30 (6) (December
2003), pp. 435–440.
11. N.A. Warner, “Natural Gas Based Direct
Steelmaking Using Melt Circulation: Technoeconomic
Feasibility,” Ironmaking and Steelmaking, 33 (4)
(2006), pp. 277–287.
12. N.A. Warner et al., “Direct Zinc Smelting with
Virtually Zero Gas Emission,” Proc. 2nd Int. Symp.
Metallurgical Processes for Early Twenty-First
Century, ed. H.Y. Sohn (Warrendale, PA: TMS, 1994),
pp. 333–349.
13. P.M.J. Gray, “Zinc Production—The Warner
Process,” Mining Magazine, 166 (January 1992), pp.
14–17.
14. P.M.J. Gray, “The Warner Zinc Process,” World
Zinc ’93 (Melbourne, Australia: The Australasian
Institute of Mining and Metallurgy, 1993), pp. 483–
489.
15. P.M.J. Gray, “The Warner Process,” Materials
World, 14 (3) (March 2006), pp. 30–32.
16. N.A. Warner, “Towards Polymetallic Sulfi de
Smelting,” Complex Sulfides: Processing of Ores,
Concentrates and By-Products, ed. A.D. Zunkel et al.
(Warrendale, PA: The Metallurgical Society, Inc.,
1985), pp. 847–865.
17. N.A. Warner, “Copper Smelting with Liquid Iron
Co-Production,” Metallurgical Processes for Early
Twenty-First Century: Volume II—Technology and
Practice, ed. H.Y. Sohn (Warrendale, PA: TMS, 1994),
pp. 351–371.
18. N.A Warner, “Conceptual Design for Lower-
Energy Primary Aluminum,” Metall. and Materials
Trans. B, 39B (2008), pp. 246–267.
19. N.A. Warner, “Co-Production of Steel and
Titanium: Process Engineering Feasibility,” Trans.
Inst. Min. Metall. C, 116 (1) (2007), pp. 34–47.
20. F. Cardarelli, “Method for Electrowinning of
Titanium Metal or Alloy from Titanium Oxide
Containing Compound in the Liquid State,” U.S.
patent application 2004/0194574 A1.
21. N.A. Warner, “Oxygen Smelting of Copper or
Nickel Sulfides,” U.S. patent 5,607,495 (1997).
22. M. Campforts et al., “On the Microstructure of a
Freeze Lining of an Industrial Nonferrous Slag,” Metall. and Materials Trans. B, 38B (2007), pp. 841–
851.
23. K.M. Donaldson, F.E. Ham, and J.G. Schofield,
“Design of Refractories and Bindings for Modern
High-Productivity Pyrometallurgical Furnaces,” Non-
Ferrous Pyrometallurgy: Trace Metals, Furnace
Practices and Energy Efficiency, ed. R. Bergman et
al. (Montreal, Canada: Metallurgical Society of CIM,
1992), pp. 491–505.
24. C.A. Rodríguez González, W.F. Caley, and R.A.L.
Drew, “Copper Matte Penetration Resistance of Basic
Refractories,” Metall. and Materials Trans. B, 38B
(2007), pp. 167–174.
25. R. Parra, R. Voytovych, and N. Eustathopoulos,
“Wetting of MgO by Cu2S-FeS Melts,” Metall. and
Materials Trans. B, 38B (2007), pp. 347–349.
Noel A. Warner is professor emeritus at the University
of Birmingham, Chemical Engineering Department,
Edgbaston, Birmingham, U.K. B15 2TT, and
can be reached at warnerna@btopenworld.com.
|