|
After World War II the United States
enjoyed a period of sustained prosperity
that enabled individual companies within
the nonferrous metals industry to finance
their own technology development. Until
the late 1970s there was a “Golden Age”
in which the original technologies of the
late 19th and early 20th centuries were
significantly upgraded and modernized.
But hard times came in the 1980s when
the costs of energy and environmental
compliance in the United States together
with the lack of anticipated growth in
second and third world economies,
and the rise of off-shore competition
plunged the industry into a depression.
Fortunately, our industry rose to the
challenge and resurrected itself—in
many ways thanks to the adoption of
new technologies and their integration
into a new global economic model. We
have now entered a new Golden Age as
the supplier of materials to emerging
economies and we are doing this by
harnessing the power of the computer
and its link to the global availability of
information.
Over the last 50 years TMS has
published a number of detailed reviews
relating to specific technology developments.
Because the topic is so broad
and in the interest of brevity, this paper
presents a general and somewhat personal
review of the history of technology
since TMS became a member society
of the American Institute of Mining,
Metallurgical, and Petroleum Engineers
(AIME).
|
THE GREENING OF THE INDUSTRY AND THE ROLE
OF RECYCLING
|
When considering the current state of the industry it should be noted that, even though we are very effective recyclers of our products, we still have to work hard on improving
our “green” image.
Because we are a mature industry operating in large visible units there is no doubt that
we have left a noticeable footprint where we have conducted our activities. It is hard not
to notice large open-pit mines and smelter stacks. Yet, when faced with the challenges of
rising environmental standards our industry has responded with massive investment which
has resulted in very noticeable benefits in nearly all the communities we work in. This has
occurred in conjunction with improving on our already good recycling track record.
The nonferrous metals industry (NFMI) has had a significant recycling component ever
since humans discovered that remelting a used or broken metallic tool was an effective
source of feed for making new implements. More recently, at the end of the 19th century,
the Guggenheims recognized the value of their scrap metal operations in Philadelphia and
grew the business into one of the original great American mining companies.
As the impact of humans on the environment became a real issue in the 1970s, the
NFMI, like everyone else, had to step up its environmental and recycling performance.
More stringent environmental regulations coming into effect at the same time the industry
was facing a downturn put severe pressure on all companies. Some, such as Anaconda,
could not stay in business and plants such as the Asarco El Paso lead smelter were no
longer economically viable. But many positive things came from the forced changes and
perhaps the best example is the closure of the sulfur loop where it is now accepted that,
in pyrometallurgical operations, SO2 will be captured and made into sulfuric acid. An
example of a modern smelter is shown in Figure 4.
In turn, this sulfuric acid can be used again by industry in applications such as leaching
oxide ores or leaching of sulfide concentrates. In hydrometallurgical applications the process
is sometimes tailored to yield elemental sulfur as a commercial product, an example
being the Sherritt Process for leaching zinc concentrates. The sulfur can then be shipped
to a new location for conversion by burning into sulfuric acid and so closing the loop.
Most metallurgical operations must also deal with fugitive emissions and water discharges.
Nonferrous smelters have always recycled their dusts internally because they
contain significant metal values. Today it is also possible to recycle such dusts in dedicated
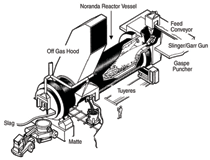
recycling facilities using robust technologies such as IsaSmelt, Ausmelt, and the Noranda Reactor (Figure 5). Another significant dust recycling technology is the use of the well-established
Waelz kiln to fume the large amount of zinc contained in electric-arc furnace
dust generated by the iron and steel industry. And in a related context, the lead industry
has had a record of fuming both zinc and lead from slags for recovery and recycling.
A variety of technologies has been developed to do this, including electric furnace and
plasma arc fuming.
The NFMI has also put a considerable effort into ensuring that aqueous discharges from
mines, mills, smelters, and refineries meet stringent standards. The most common technology
used is simple alkaline precipitation of heavy metals, usually with lime or sulfide, and
subsequent recycling of the precipitates to smelters or solid waste treatment plants. These
precipitates or sludges are often excellent feeds for smelters employing technologies such
as IsaSmelt or AusSmelt or the Noranda Reactor because they can accept moist, variable
feeds. Often technologies such as high-density separation and reverse osmosis are used
to improve the effectiveness of metal removal from the discharge streams.
Another closed loop has steadily evolved over the years with lead. Because of understandable
environmental concerns, lead has essentially evolved into a one-market metal—in
lead-acid batteries. The components of these batteries are fully recyclable—lead metal
alloys, lead oxide paste, plastic separators and cases and, of course, the sulfuric acid
electrolyte. Recycling rates for batteries now run in the high 90 percent range and the
public has no reason to discard batteries irresponsibly. This means that the lead industry
in the developed world is now totally dominated by the secondary (recycling) industry.
Technology development for lead has focused on the need to be able to accept the variable
feeds coming from the recycled batteries.7
Higher-value metals, such as nickel and cobalt, when used in products such as nickel-cadmium
and lithium-ion batteries, can be valuable sources of feed to nickel smelters.
Even higher value metals such as precious metals and platinum group metals (PGMs)
have always had high recycling rates. Precious metal scrap, especially from the electronics
industry, is aggressively sought by primary copper smelters such as the Horne (Canada)
and Umicore (Belgium), which have elected to install flexible feed technologies such as
the Noranda Reactor and IsaSmelt, respectively.
Nickel smelters are useful conduits for the recycling of PGMs because their downstream
refineries are already set up for recovering these elements. Electric furnaces such as the
Xstrata furnace at Sudbury in Canada can readily accept PGM feeds. Sometimes PGMs
are in a discrete enough form, such as in automobile catalytic converters, that a standalone
technology like plasma smelting can be used in a dedicated plant.
|
FROM WORLD WAR II TO THE GOLDEN AGE
The nonferrous metals industry
(NFMI) as we currently recognize it
essentially came together in the late
19th and early 20th centuries. It was the
early corporate giants from this era such
as Asarco, Phelps Dodge, Anaconda,
etc., which supplied the metals and
materials to America as it developed its
infrastructure on the way to becoming
the world’s preeminent economic power.
The numerous technologies initially
developed for metallurgical processes
were characterized by the personal
attribution given to the inventors, such
as the Peirce-Smith converting of mattes
and the Betts process for refining lead.
All of this changed in North America
after World War II. Since there was no
war damage to overcome, the economy
came out of the Great Depression and
took off. The concept of suburban living
as being ideal for families grew enormously.
This led to huge expansions
in home building, new appliances for
these homes, multiple automobiles for
one family, and the ability to travel long
distances by car and plane.
The NFMI reflected these expectations,
with many companies expanding
their activities to improve their profits by
including downstream and added-value
products—a “soup-to-nuts” approach.
The desire to integrate downstream resulted in the formation of operations
and subsidiary companies to produce
refi ned by-product metals, specialty
alloys, metal powders, advanced materials,
custom chemicals, etc. Indeed, the
author, who has a historical background
in this area, has frequently given a presentation
titled “The Production of Minor
Metals—or How to Get the Squeal out
of the Pig.”
Companies were greatly assisted at
this time in their technology development
by the quality of their labor force,
many of whom were funded in their
education by the G.I. Bill. When these
people entered employment following
university they also were joining well-established
companies with guarantees
of long-term employment. When the
author came into the industry in 1974
the majority of his senior co-workers
were coming to the end of such careers
and they were able to internally provide
vast knowledge and experience as well
as mentor newcomers. The stability of
the work force and its role in technology
stands in stark contrast to the situation
today where there is often no corporate
collective internal memory and knowledge
must be gleaned from outside
sources.
So what exactly is the technological
legacy of the period from World War II
to the late 1970s and why is it considered
to be a Golden Age, as first defined by
Nicholas Themelis in 1993?1
Most of the physical plants for the
NFMI industry built in the early days of
the industry were in need of technological
retrofits and upgrades. Many companies
were financially strong enough to set up
corporate R&D centers to provide them
with the technologies they would need
to grow their businesses. Typical of this
genre were the technology centers built
by Asarco (New Jersey), Amax (Colorado),
and Kennecott (Massachusetts and
Utah) as well as the Noranda, Falconbridge,
and Inco facilities in Canada.
The mandates of these centers varied.
At one end of the spectrum there were
the blue-sky hopes of the Ledgemont
Laboratory, built by Kennecott, at a location
remote from operations and using
a model loosely based on the Bell Labs
concept—put enough intelligent people
together and they will invent something
useful. At the other end development was
done at or close by operational sites. But
it was accepted that technology development
would be driven internally from the
corporate technology “silo.”
So the expected happened. Faced
with the same technical challenges of a
relatively mature industry, each company
came up with its own solutions. This
resulted in the emergence of a wide
variety of technologies for the generic
problems of the industry, particularly the
need to process declining ore grades in
North America.
In his address to the AIME on its
centenary in 1970,2 Herb Kellogg noted
that technological progress fell into
three categories: The Bigger and Better
Process, The New Process by Virtue
of Engineering Design, and The New
Process by Virtue of Novel Chemistry.
Not surprisingly, as we review progress
in 2007, Kellogg’s characterization has
stood the test of time.
His category of the Bigger and Better
Process was linked, as noted previously,
to the fact that the overall grades
of ore available to the mature North
American industry had started to fall.
More tonnes of ore had to be mined
and milled to sustain production tonnages
of metals. There was a matching
need to increase throughput to lower
the unit cost of production and this was
most easily achieved by increasing the
intensity of the processes, improving
mechanization—greatly assisted by the
introduction of the first microelectronic
devices—and increasing the physical
size of the plants.
An illustration of the Bigger and Better
Process is the Amarillo Copper Refinery
(ACR) opened by Asarco in 1975. The
refinery capacity was set at 480,000 t/y
and it replaced three refineries built in the
late 19th century with a combined capacity
of about half that of ACR. With respect
to a better process, almost immediately
after ACR was commissioned, Mount
Isa Mines and Kidd Creek realized that plating a sheet on stainless steel could
be continued for at least seven days
to make directly saleable cathode and
eliminate the need to make a precursor
one-day starting sheet. Since the early
1980s this is the only technology used
in new copper refineries.
In smelters the need for energy
efficiency and improved hygiene was
becoming apparent. The industry recognized
that operations such as the
roaster/reverb smelting used for copper
concentrates were not economical for
the handling of much larger tonnages of
concentrates. The change began when
Outokumpu and Inco started work on
the concept of flash smelting in the
1940s. The need to intensify the smelting
process was further addressed by
the addition of oxygen injected through
burners, tuyeres, or lances. This was
technology transferred from the basic
oxygen furnace developed for the steel
industry.3 At the same time, furnace life
was greatly improved by the adoption of
cooling technology within the furnace
refractories. A variety of new smelting
technologies emerged. The Inco and
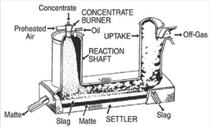
Outokumpu (Figure 1) technologies
are characterized by the injection of
oxygen through the concentrate burners
whereas Mitsubishi (Figure 2) and
IsaSmelt technologies take advantage of
injection lances to increase smelting rates
by agitation of the bath. The Noranda
reactor and the El Teniente converter,
developed in the same time period,
exhibited another variation of process
intensification by injecting concentrates
through submerged tuyeres. All of these
technologies have driven up the copper
grades of the mattes being received by
the converters, thus enabling them to
match the increased capacities of the
smelting furnaces feeding them.4
These smelting technologies, which
rapidly became commonplace in the
1970s and 1980s, soon moved into the
second phase identified by Kellogg as the
New Process by Virtue of Engineering
Design. The advent of smelting concepts
such as the Noranda Reactor (for copper)
and the QSL and Kivcet reactors (for
lead) represented attempts at using a
single reactor for the entire smelting
process. Although the Noranda Reactor
has not evolved into a single-step commercial
process for copper, both the QSL
and Kivcet reactors are now used commercially
for lead smelting. The Teck
Cominco Kivcet reactor at Trail, British
Columbia, stands out as a testament to a
process where lead concentrates are fed
to the furnace and slag-free hard lead
bullion is tapped from the same reactor
as a final product.
In the zinc and nickel industries the
third category of development—New
Processes by Virtue of Novel Chemistry—
has appeared.5 Traditionally, zinc
sulfide concentrates were roasted to
oxide and then leached. In 1981, Sherritt
Gordon together with Cominco developed
a process (Figure 3) to pressure
leach the concentrates to dissolve the zinc
and convert the sulfur to its elemental
form for recovery. The first plant was
installed at Cominco, Trail, in 1981.
For nickel, the challenge was of a
different nature in that most the world’s
nickel resources became lateric (oxide)
ores versus the sulfide ores which had
been the mainstay of the industry since
the early 20th century. Lateritic processes
were originally developed for Cuban
ores. The Caron process, a pyro/hydro
hybrid, was introduced as the first commercial
process in the 1940s and was
followed by pressure acid leaching in
the 1950s. These processes required
a hydrometallurgical back end so that
nickel and cobalt in limonitic ores could
be separated. In the 1960s and 1970s the
focus switched to saprolitic ores which
contain little cobalt but are high in acid-consuming
magnesium. As a result, fully
pyrometallurgical processes emerged,
especially the rotary kiln electric furnace
(RKEF) process which dominates today.
The saprolitic processes send reduced
nickel and partially reduced iron to
electric furnaces for final reduction to
metal as ferronickel.
Interestingly, the current challenge
facing the lateritic nickel industry is
how to get Bigger and Better to meet
world demand for nickel. The inability to upgrade laterite ores handicaps
development of new processes which
are now required to be energy efficient
and meet stringent global environmental
standards. Currently, there is no limonitic
plant with a capacity of >55,000
t/y and the largest saprolitic plants still
produce <100,000 t/y nickel. The main
reason for this lies, of course, in the
fact nickel in laterites is locked in solid
solution and cannot be beneficiated like
its discretely mineralized sulfide cousin.
Laterite plants must process feeds containing
only 1–2% nickel whereas some
smelters may see sulfide feeds as rich as
18% nickel. The nickel laterite industry
stands at a crossroads as it struggles to
bring on significant new capacity for
such low-grade feeds.
In summary, from the end of World
War II to the early 1980s, the NFMI
had a wide range of options if it either
wanted to upgrade a plant or install a
greenfield operation. The reader will
have noted a decided trend in the text
so far—a “silo” model of technology
development reflected in the fact that
new process names came directly from
the companies that invented them.
Typically this is reflected in the process
nomenclature arising from this period
such as:
- Outokumpu, Inco, Mitsubishi,
Noranda, IsaSmelt, QSL (in part)
smelting processes for sulfide
concentrates
- Isa Process, Kidd Process for the
direct plating of copper
- Sherritt Gordon and CESL
leaching processes for sulfide
concentrates
- Asarco shaft furnace for copper
cathode and anode melting
There are a reasonable number of
North American names in this list but
the international component is also very
high. This indicates that globalization
was starting to impact the industry by the
1970s. At that time the business model
was for companies to have overseas
affiliates operate as separate entities with
little communication with the parent
company. But the international value of
technology transfer was becoming noted
and by the early 1980s companies such as
Outokumpu, Mount Isa, and Mitsubishi
were capable of marketing worldwide.
As the 1970s came to a close the NFMI
was in reasonably good shape. The oil
industry, cash rich due to the high price
of oil, viewed base metals companies
as places to invest. New owners such as
Exxon, Atlantic Richfield, and Sohio
joined the ranks with such long-established
companies as Phelps Dodge,
Asarco, and St. Joe Minerals. In the
short term there was a technological
stimulus due to the (ironical) need for
NFMI companies to become much more
energy efficient now that energy costs had
risen significantly. Also, the needs for
environmental compliance were becoming
significant and companies needed to
spend capital for sulfur dioxide capture
as well, ensuring that atmospheric metals
emissions and aqueous discharges met
the new standards.
But it turned out that storm clouds
were on the horizon and there would be
a significant delay before the NFMI truly
benefited from the Golden Age.
THE 1980s—A DECADE OF GLOOM
At the start of the decade NFMI
companies were doing well due to the
consequences of high inflation. As noted
previously, there was ongoing recapitalization
of the NFMI with commitments
to installations such as the Inco flash
furnaces at Chino and Hayden, the Mitsubishi
furnace at Kidd Creek, Sherritt
Process installations at Trail, Kidd Creek,
and Hudson Bay, and the Outokumpu
flash furnace at Hidalgo. Also the need
to strive for much better environmental
compliance meant that companies had
started to invest significant dollars in SO2
capture in the form of sulfuric acid plants.
Aqueous discharges—both mine tailings
and smelter and refinery eluants—were
of concern and water treatment became
mandatory at many NFMI sites.
The investment in technology at corporate
research and technology centers
helped to mitigate the impact of higher
costs of energy prices and environmental
compliance. However, the industry
could not fight off indefinitely the effects
of the recession in the early 1980s, allied
to the lack of predicted growth in less developed
nations. There also has been
a number of events such as the nationalization
of the Chilean copper industry
which discouraged fresh capital investment
in the NFMI at this time. The
industry went into a prolonged swoon, from which, at one time, it looked like
the North American companies would
not recover. Metal prices plunged and in
1984 Business Week magazine proclaimed
the “Death of Mining” on its
cover.6 It also became apparent that the
United States was now in serious competition
with the lower cost of metals
production in various places around the
globe, especially in countries such as
Chile, Mexico, and Peru.
By 1985 the NFMI in the United States
was in deep trouble. Companies were
near bankruptcy and senior managements
all over had cut costs to address
the issue by conducting fire sales of
assets, closing plants, and reducing
corporate overhead in functions such as
technology centers. Whereas the 1950s
and 1960s saw the rise of the corporate
technology center, the 1980s saw their
wholesale decline in North America.
Also the oil companies, flush with cash
at the start of the decade, started to bail
out of their “underperforming assets,”
which only worsened the situation.
The deep gloom persisted from 1983
through 1987, when the first signs of
revival appeared. Interestingly, this came
from companies that had kept faith in
technology. Although formal corporate
support of technology was no longer
strong, the legacies of the Golden Age
came to the rescue. Phelps Dodge, a
company which appeared headed for
bankruptcy and closure in 1984, invested
in heap leaching/solvent extraction
technology for the unique chalcocite
mineralogy of the Morenci mine. They
were able to turn the fortunes of the
company around in a few years. By the
end of the decade, Phelps Dodge had
returned to its preeminent position in
American copper producers by extending
this low-cost option for producing high-grade
copper to a number of heap-leaching
operations.
Magma Copper invested in Outokumpu
flash smelting at San Manuel and
eliminated the last major SO2 output from
a U.S. copper plant. Other bold steps in
technology taken at this dark time
included Cominco’s installation of QSL
smelting at Trail (later to be switched to
Kivcet) and BP’s multi-phase investment
in Kennecott Utah, where it completely
revamped the process from mine to
refinery by installing the following
technologies: large-scale open pit
mining, large-scale semi-autogenous
grinding milling, installation of a 1 million
t/y capacity smelter with both flash
smelting and decoupled flash converting,
permanent cathodes for copper electrorefining, and more than 99% sulfur
dioxide capture. Other companies followed
suit and by the end of the decade
further commitments were on the horizon,
such as the Cyprus IsaSmelt reactor
at Miami and the Asarco ConTop cyclone
smelting process at El Paso. In addition,
Noranda adapted its single blister-making
concept to a large-capacity
process producing very high grade (75%)
matte.
By the early 1990s the U.S. NFMI
was in much better shape, although nickel
was adversely impacted by the collapse
of the Soviet Union and the accompanying
flood of stainless steel scrap that
came on the market. However, significant
permanent change had occurred in how
companies viewed technology development.
Most companies had begun working
as integrated worldwide entities,
assisted by the beginnings of true globalization.
In part, this was due to the
advent of much improved communication
arising from the microelectronic
revolution, manifested in the form of
satellite telephones, telefaxes, and ultimately
the introduction of e-mail and
the Internet. Using these tools, global
information exchange became available
to everyone in the industry and gave
every company the ability to develop
“virtual technology” concepts.
The NFMI also moved strongly to
take advantage of economies of scale.
Mines became larger, thus driving down
the cost of the most expensive part of our industry—mining and milling. The
increased outputs from such operations
forced smelters and refineries, in turn,
to increase capacity.
Fortunately, the technological legacies
of the Golden Age allowed the downstream
operations to match this change.
In two decades the industry moved from
smelters typically producing 100,000
t/y of copper in roaster/reverb operations
to flash and bath smelters, some with the
capacity to treat in excess of 1 million
t/y of concentrates producing over
300,000 t/y of copper. Copper refineries
expanded to 500,000 t/y or greater capacity.
Their production was greatly helped
by permanent cathode technology being
expanded to 10–14 day plating cycles
and by reagent control technologies such
as Reatrol® and Collamat.® Now current
efficiencies in copper tankhouses are
often 95% or better.
In the zinc industry the invention of
the jumbo cathode by Vielle Montagne
in 1969 led to the building of refineries
with 250,000 t/y or more capacity. This
came about by the development of
mechanical stripping which decoupled
production from the size of a plating
sheet capable of being handled by an
individual. Instead of hand harvesting
after 1 day, some zinc tankhouses can
mechanically harvest three-day jumbo
cathodes from sheets with three times
the surface area of the manual processes.
For nickel, investment switched primarily
to the building of plants for processing
laterites by the RKEF method
although there was also investment in
the flash smelting of sulfides by Inco and
Outokumpu.
Only the lead industry, which saw its
focus change overwhelmingly to treating
secondary materials from batteries, did
not see the same level of investment in
new technology.
See the sidebar for environmental
developments in NFMI.
THE PRESENT
The late 1990s/early 2000s saw the
NFMI once more in a depressed state.
A global economic downturn sparked by
such events as the Asian currency crisis
and the prolonged Japanese recession put
the NFMI in a state of surplus capacity
for most metals. But luckily, the Chinese
Dragon was about to wake up!
The demand from China for all
commodities brings to mind an experience
of the author in 1981. In that year
Charles F. Barber, then the chairman of
Asarco, pronounced in a farewell speech
that metals are “the building blocks of
civilization.” It turns out that Barber was
correct because, of course, the Chinese
are now in the process of building their
industrial civilization using our metals
as building blocks.
Our operations are stretched to capacity
and prices have risen to unprecedented
levels. Ironically, this has brought with
it the paradox that costs of materials for
new NFMI plants, as well as the contingent
engineering services, have risen to
the point that greenfield installations
need to be justified on totally different
economic parameters than from just 5
years ago. When the author entered the
nickel industry in 1998, a 66,000 t/y
nickel laterite plant was predicted to cost
$1.5 billion to build ($12/installed annual
lb.). Such a plant could meet a 15%
hurdle rate at long-term nickel prices
between $3–3.50/lb. The economics of
such plants have doubled since then and,
in 2007, companies are being required to
risk capital based on nickel prices which
now must clearly average $6–7.00/lb. for
the life of the project—prices which are
currently realizable but have never been
seen on an extended basis.
So how are we going to resolve this
paradox and move to even better times?
Simply, we need to be smarter and harness
our metallurgical and mechanical
engineering needs to the virtual world
of the microelectronics engineer. If this
is done successfully the best may be yet
to come for our industry.
THE FUTURE—IS THE BEST STILL TO COME?
Consider so far the impact of the electronic
age on our industry. This article has
already noted our ability to communicate
effectively on a global basis. Global
standards of engineering can be easily
implemented no matter the location of
the operation or project. The technology
of the high Andes is the same as that of
the Indonesian jungle as the plains of
Western Australia. The availability of the
Internet means that engineers have ready
access to the same level of information
no matter where they work.
What information are we talking
about? We are referring to tools such as
mathematical modeling of processes
with techniques such as MetSim, Aspen
Plus, and FactSage, which can access all
the electronic data bases that have been
set up. This does not mean the technical
librarian and corporate memory are no
longer needed but instead that all of this
knowledge can be combined to greater
effect.
The ability to use information electronically
means we can try and test our
thermodynamic, kinetic, mechanical,
and materials of construction assumptions
before we do a major test. Design
of experiments minimizes the costs of
the actual test programs and also maximizes
the information to be gleaned.
There is frequently a mandate to produce
data from such tests which will meet a
95% confidence limit. We can move
forward to the piloting, demonstration,
design, and detailed engineering phases
of technology implementation knowing
that we have identified a robust process.
The additional value of inserting maintenance
and materials issues in to the
virtual design process cannot be over
emphasized.
Similarly, the value of process control
must be recognized. In the 50 year time
span being reviewed in this paper, process
control has emerged from its infancy in
instrumentation—sensor alarms, level
controls, etc.—to the much higher levels
of operational prediction and performance
optimization. An example is the
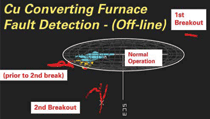
use of multivariate analysis techniques8
in smelters to alert operators when furnace
run-out conditions are being
approached. Figure 6 illustrates how a
large data set derived from thermocouples,
particularly at the bath level, is used
to set boundary conditions relating to a
safe operating mode and predict the onset
of run-out conditions. Expert Systems
is another powerful tool to help operators
run their plants at optimum levels.
Since our industry no longer possesses
the technical depth it once retained in its
corporate organizations, we must be even
more aware of what the outside world
has to offer. As the fluid bed reactor was
brought in from the petroleum industry
to replace tray and hearth roasting, so
we must look elsewhere for more novel
technologies. Both pyrometallurgy and
hydrometallurgy are forms of applied
chemistry and we can expect the world
of chemical research to bring us new developments. An example is the use of
computational stereochemistry to create
essentially designer chemicals for hydrometallurgical
applications. Two such
technologies are the application of crown
ether molecules (already commercial)
and zwitterionic ligands to selectively
extract targeted ion pairs from solutions.10
We are just beginning to see the impact
of the biological revolution on our industry.11 It has long been known that bacteria
greatly impact heap leaching. The
ability to develop new strains of bacteria
which can resist higher temperatures and
toxic impurities in ores is slowly but
surely impacting our industry.
As we look forward to a bright future
a word of caution is inserted. As powerful
as the computer is and the accompanying
virtual world, there is still no
substitute for an engineer who has a grasp
of the real world. Engineers must be able
to work in a plant environment so they
can develop instincts on how things are
working. It is similar to the advice that
the author received when solving mathematical
problems as a student—does
the answer make sense? If not, why
not?
The NFMI will continue to operate
smarter, to be more energy efficient, and
to work to meet ever more stringent
environmental standards. At some point
we can expect to see the next generation
of breakthrough technologies that will
impact our industry. Mostly likely they
will either be seen in the mining sector,
which incurs the highest part of the cost
of producing metal, or the milling sector,
which incurs the greatest losses of metals
values to reject tailings.
The signs are there. In mining the
efforts to develop in-situ mining of the
1990s such as the copper leaching processes
in Arizona at Casa Grande,
(USBOM, Asarco, and Freeport McMoran)
and Florence (BHP) will likely be
revisited because the concept is elegant.
The process leaves no footprint above
ground and the pregnant solution produced
can immediately go to a refinery.
We can also look forward to increasingly
automated mining which will allow us
go to areas, particularly at a depth where
ground stability currently makes it unsafe
for human miners. Many ore bodies are
richer at depth (e.g, Cu/Ni in Sudbury,
Ontario and copper at Superior, Arizona).
In milling, technologies such as ultrasonic
comminution may significantly
drive down milling energy costs. This
may be allied to synergistic technologies
such as advanced process mineralogy.12
This technology is today based on the
advanced quantitative capabilities of
QEM*SCAN® and MLA® analyzers
which can accurately measure the mineralogy
of thousands of particles in a
sample. In turn, the data can be used with
mathematical certainty to develop virtual
flowsheets which identify the optimal
grinding sizes needed to recover pay
metals locked in the mineral grains.
Additionally, this can be further linked
to ultrafine grinding technologies which
have overcome the problem of fine slimes
flotation. All of this results in the much
improved economic performance of
milling circuits. Downstream smelters
and hydrometallurgical plants can now
receive higher-grade feeds with much
less metal value being lost to the tailings
dam. So getting the “squeal out of the
pig” is being moved back in the process
stream from the refinery to the mill.
CONCLUSION
With the prospect of a Supercycle in
the years to come, the NFI has the ability
to serve the world even more effectively,
particularly if it harnesses the
power of the microelectronic revolution
and applies it to novel technology development.
As a final comment, this account of
technology development over the last 50
years serves as a testament to an industry
which often does not advance its
image as much as it could. Nonferrous
metals production is a high-tech business
requiring advanced understanding of
thermodynamics and mathematics to
make pyrometallurgical, hydrometallurgical,
and electrochemical processes
feasible. Often these processes must be
carried out in extremely hostile environments
and the selection of materials to
contain and sustain the processes is a
tremendous challenge.
In the time period reviewed, the NFMI
has managed to significantly improve
the quality of our products and dramatically
lower the costs of making our
metals. This has been accomplished
despite the ever-rising price of energy
and always within the constraints of the
commodity-based economic system
which does not allow us to sell our
products at prices reflective of true costs.
At the same time, this has been done
while recapitalizing our operations so
that they also meet the requirements set
for the environment and for sustainable
development in the communities where
we work. The contributions of all those
who have worked in technology development
during this time period truly deserve
recognition.
ACKNOWLEDGEMENTS
This paper is based on the keynote
presentation given on behalf of the
Extraction & Processing Division in
recognition of the 50th Anniversary of
TMS joining AIME. The author gratefully
acknowledges the many conversations
over the years with members of TMS
which contributed input to the paper
and, in particular, the specific support
and comments from Phil Mackey of
Xstrata Nickel and Sam Marcuson of
Companhia Vale do Rio Doce.
REFERENCES
1. N.J. Themelis, Vuoritellisuus Bergshanteringen, 51
(2) (1993), pp. 90–95.
2. H.H. Kellogg, AIME Centennial Volume, 1871–1971
(New York: AIME, 1971), pp. 147–161.
3. P.J. Mackey and J.K. Brimacombe, Savard/Lee
Intern. Symp. on Bath Smelting, ed. J.K. Brimacombe
et al. (Warrendale, PA: TMS, 1992), pp. 3–28.
4. N.J. Themelis, JOM, 46 (8) (1994), pp. 51–57.
5. R.M.G.S. Berezowsky et al., JOM, 43 (2) (1991), pp. 9–15.
6. “Death of Mining,” Business Week (17 December 1984).
7. M. King and V. Ramachandran, Encyclopedia of
Chemical Technology, 4th Edition, Volume 15, ed. Kirk
Othmer (New York: John Wiley and Sons, 1995), pp. 69–113.
8. P.E. Thwaites et al., “System and Method for Furnace
Monitoring and Control,” Canadian patent application
2,469,975 (4 June 2004).
9. R.L. Breuning et al., Hydrometallurgy 2003, Volume
1: Leaching and Solution Purification, ed. C.A. Young et
al. (Warrendale, PA: TMS, 2003), pp. 729–739.
10. S.G. Galbraith et al., Hydrometallurgy 2003,
Volume 1: Leaching and Solution Purification, ed. C.A.
Young et al. (Warrendale, PA: TMS, 2003), pp. 941–954.
11. H.R. Watling, Hydrometallurgy, 84 (2006), pp. 81–108.
12. D. Fragomeni et al., Proceedings of the 37th Annual
Meeting of The Canadian Mineral Processors (Montreal, ON, Canada: CIM, 2005), pp. 75–98.
Michael G. King retired as the Director of Metallurgical
Technology for Xstrata Nickel (formerly Falconbridge
Ltd.). Mr. King can be reached at 806 N.
Northpoint Drive, Salt Lake City, Utah 84103-3346;
e-mail michaelking806@comcast.com
|